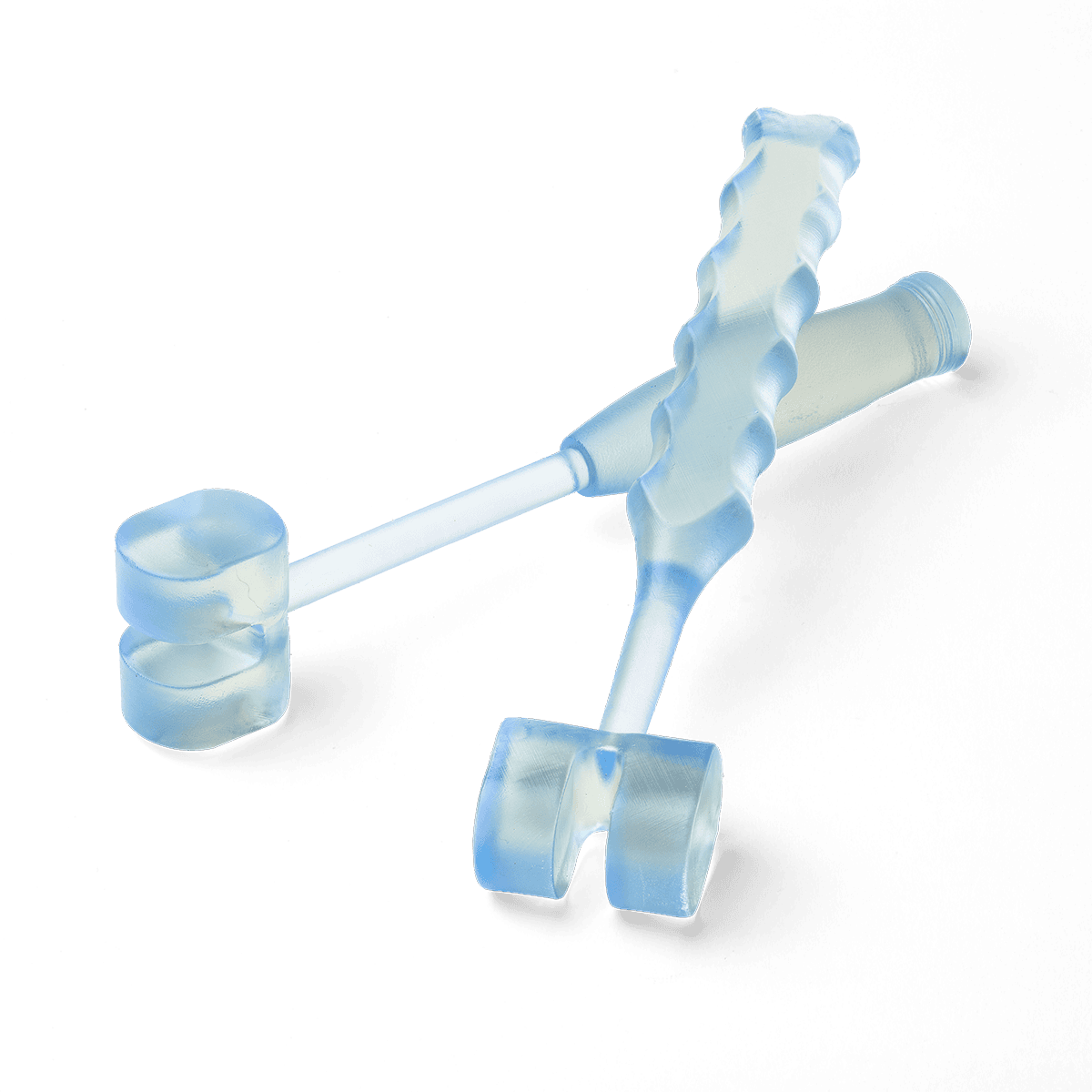
Why Choose BioMed Durable Resin?
Fuel medical innovation using 3D printing and BioMed Durable Resin for producing end-use devices where performance, impact resistance, and biocompatibility are critical.
Impact, Shatter, and Abrasion Resistant
Produce medical-grade parts that are impact resistant with a Notched Izod of 98 J/m.
Certified Medical-Grade Material
Leverage a material manufactured within our Quality Management Systems, with strict adherence to ISO 13485.
Biocompatible Parts
Common use case testing by Formlabs is intended to instill confidence that this material is suitable for biocompatible applications.
Excellent Clarity and Surface Finish
Parts produced using this material look and feel like end-use parts and have a very smooth surface finish.
Applications*
Introduce cutting-edge patient-specific instruments (PSI) and procedure-specific medical devices at the point of care. Improve patient outcomes, reduce complications, and reduce operating time with medical devices printed in BioMed Durable Resin.
BioMed Durable Resin is ideal for:
*Unlike Formlabs Dental Resins, no intended uses are provided for BioMed Resins. Material properties may vary based on part design, manufacturing practices and other methods. It is the manufacturer’s responsibility and its respective customers and end-users to determine the biocompatibility and performance of all printed parts for their respective application and use. To instill confidence in potential users, Formlabs has tested biocompatibility and sterilization compatibility for common use cases and manufactures BioMed Resins in an ISO 13485 certified facility. We can offer representative examples of what customers have done with our products and the supporting documentation we make freely available.

Formlabs manufactures our Biomed and Medical Device Resins at our FDA Registered facility in Ohio. These materials are designed and manufactured within our robust Quality Management System that is ISO 13485 and EU MDR certified. A dedicated team of operators and quality assurance professionals make the resins inside a certified ISO Class 8 clean room. All of our Medical Device Resins are appropriately registered with FDA and CE marked according to the EU MDR. View our ISO 13485 certification.
Material Properties*
BioMed Durable Resin
Ultimate Tensile Strength
Tensile Modulus
Elongation at Break
Flexural Strength
Flexural Modulus
Hardness Shore D
Notched Izod
Heat Deflection Temp @ 0.45 MPa
*Material properties may vary based on part geometry, print orientation, print settings, temperature, and disinfection or sterilization methods used. Data were measured on post-cured samples printed on a Form3B with 100um BioMed Durable Resin settings, washed in a Form Wash for 10 minutes in 99% Isopropyl Alcohol, and post-cured at 60°C, 20 minutes in a Form Cure. BioMed Durable Resin was tested at NAMSA World Headquarters, OH, USA.