Protective Face Shields Against COVID-19
What are Face Shields?
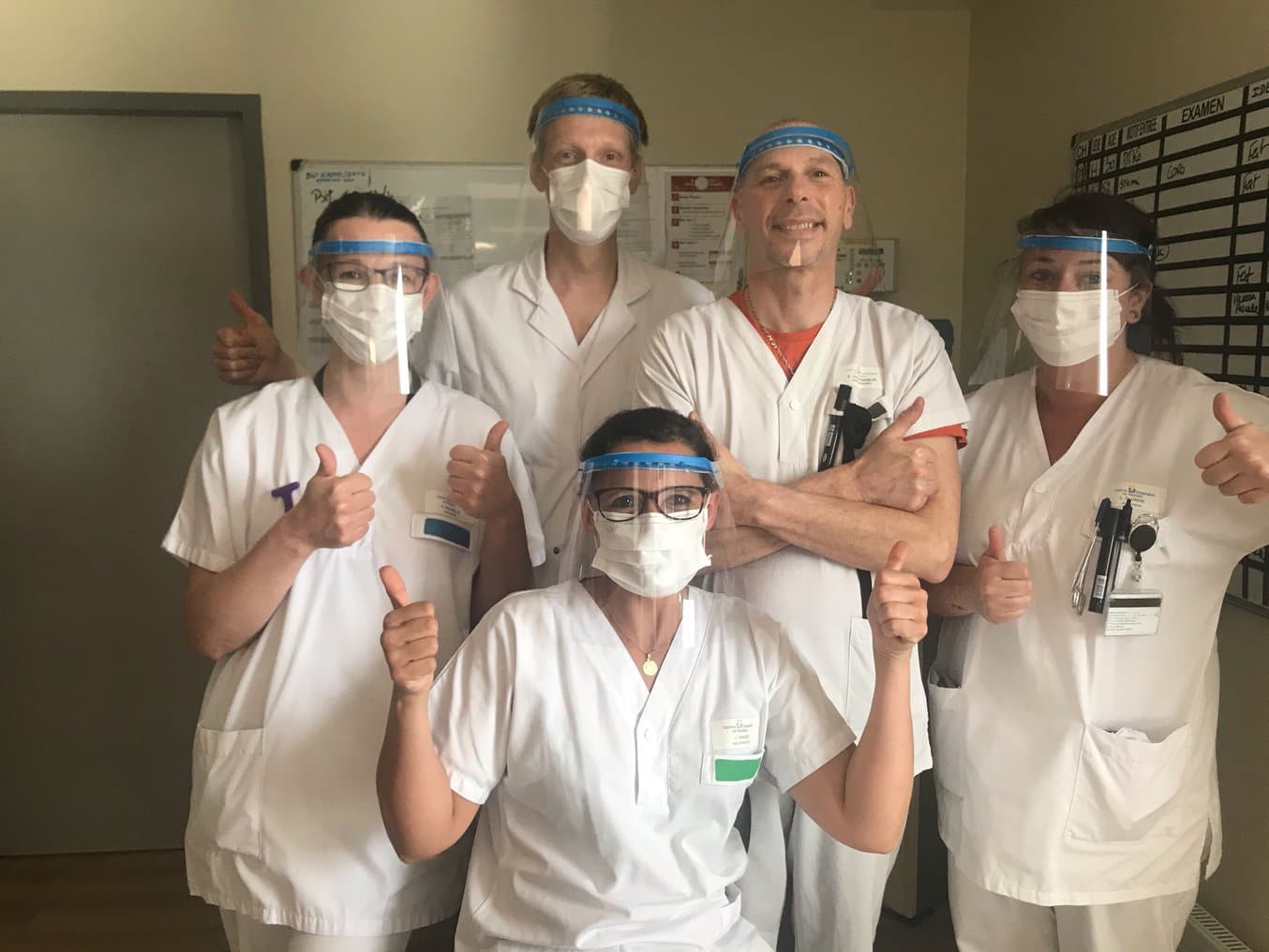
Medical workers with face shields printed by So-boat.
Problem
Frontline healthcare workers are running out of personal protective equipment (PPE). Many hospitals have a limited supply of PPE or have run out, leaving healthcare professionals without the necessary resources to perform their job safely and raising their chances of infection.
This page describes efforts originating in the US. Find out more about the development of mask adapters in France.
Solution
Disposable plastic face shields are intended to be worn over a healthcare worker's mask to protect against patients' coughs, sneezes, and sweat.
Face shields limit aerosol and splatter exposure from the front, protecting a broader area of the face compared to standard safety goggles or glasses, while providing top ventilation. By reducing aerosol and splatter exposure on N95 and other face masks that cover the mouth and nose, shields also allow healthcare workers to wear those masks longer.
Face shields consist of a transparent shield, a horseshoe-shaped strip of plastic that serves as the frame of the shield, and an elastic band to wrap around the head. The horseshoe-shaped strips can be 3D printed, while the rest of the parts are widely available off the shelf components.
Face shields are one of the most popular applications of 3D printing to create PPE.
Important Note on Material Selection

Biocompatible materials should be used for applications requiring skin contact (see disclaimer). Formlabs Surgical Guide Resin, indented for printing endosseous implant accessories, is biocompatible for short term surface contact (ex: mucosal membrane) and may be autoclaved.
If a more pliable material is used to increase comfort, such as Formlabs Tough 1500 Resin or Durable Resin, we recommend the application of biocompatible coatings (e.g., luxaprint flex coat and ComfortCoat) or adhering moleskin tape across any piece which may touch the skin.
Make sure the biocompatible coatings are also compatible with other application requirements prior to use. An example is EP42HT-2Med, a USP Class VI coating that is solvent resistant and compatible with multiple sterilization cycles. Follow manufacturer instructions to ensure uniformly coated surfaces for optimal performance.
Note: Luxaprint flex coat, ComfortCoat, and EP-42HT-2Med are third party coatings not affiliated with Formlabs Inc.
Custom Face Shields in Action
Formlabs User Luiz Maracaja on Draft Resin
“[Draft Resin] is a game changer for prototyping, testing and reaching the final design.” - Luiz Maracaja
Formlabs user Luiz Maracaja worked with his colleagues at Yale School of Medicine and University of Mississippi to devise a novel face shield frame called OxyFrame. The 3D printed device combines a traditional face shield framework with a novel air channel system that allows inflow of oxygen or compressed air behind the ear and outflow above the nose-bridge.
Using OxyFrame, continuous air flow allows the provider to breathe comfortably inside a plastic hood, while simultaneously defogging the clear shield film. The design is specifically optimized to print vertically on an SLA printer with minimal supports, enabling users to tile 24 frames onto one build plate. Each frame uses only 9.7 mL of the resin material, so one liter of the resin can generate approximately 100 frames.
Luiz Maracaja and his colleagues prototyped their design using Draft Resin. Prints in Draft Resin provided sufficient surface quality while printing with remarkable speed and efficiency; the team was able to print ten prototype frames in three and a half hours.
To learn more about the work Luiz Maracaja and his team are doing, along with other non-medical parts being created to combat the COVID-19 crisis, read about our Community Parts Library.
Validated 3D Printed Designs
Formlabs has investigated and trialed numerous face shield designs over the past two weeks. As of this update, we are aware of two 3D-printed designs validated for clinical use by NIH.
The transparent visor is not 3D printable and may be available from traditional manufacturers and medical suppliers. The transparent plastic is often available in sheets and laser cut. NIH is now allowing the use of standard US letter-sized transparencies (8.5x11 in).
Currently Available Face Shields:
- Printable on Formlabs Printers: IC3D Budmen Face Shield
- Printable on non-Formlabs Printers: DtM-v3.0 Face Shield
Disclaimer
Formlabs has created this website and has become involved in varying degrees with the projects described here during a global medical crisis. Formlabs is working on many projects to address global healthcare-related supply shortages around the world, but in trying to mitigate these shortages, patient safety is still Formlabs’ top concern. We must remind those who are helping to alleviate these shortages that masks, swabs, face shields, and other 3D printed products intended to prevent or treat COVID-19 are medical devices. These devices must be safe for their intended purpose and anyone considering the manufacturing of these products should consider the following items:
-
Formlabs is a manufacturer of 3D printing materials capable of fabricating finished devices according to their labeling and any other product manufactured from these materials should be verified and validated according to their intended purpose.
-
You may be fabricating a device that does not have the required regulatory approvals and clearances. If you are fabricating devices, follow the guidelines on the label for each material. You may seek to obtain reliable regulatory advice.
-
Please consider local regulations, material safety data sheets, software capabilities, sterilization requirements, and institutional requirements before 3D printing medical devices.
-
Regulatory agencies (such as the FDA) may consider expedited review of manufacturing information and/or premarket submissions.
Formlabs cannot warrant that any products not manufactured by Formlabs are suitable for their intended purpose.