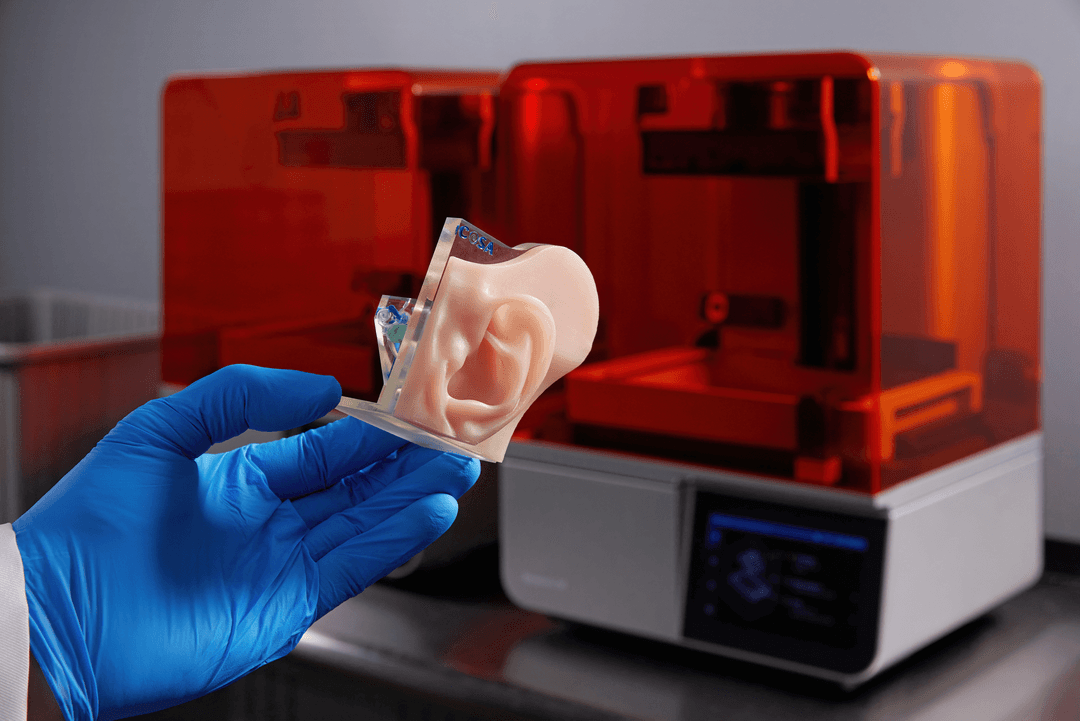
Developing a medical device can be a long and expensive process, encompassing years and hundreds of thousands of dollars. The Formlabs Regulatory Affairs and Quality Assurance (RAQA) team works with Formlabs users to help streamline this process. One way to do this is by providing Master Files for Formlabs BioMed Resins to the US Food and Drug Administration (FDA).
“The confidence [that the Master File gave us] made it a $150,000 510(k) as opposed to a $500,000 510(k). This wouldn’t be as good of a story without such a solid Master File to start with.”
Mauricio Toro, co-founder and CEO, TechFit
What Is a Master File?
A Master File is a confidential submission to the FDA that contains information that can be referenced by other companies in their pre-market submissions, including Premarket Approval (PMA), 510(k), Investigational Device Exemption (IDE), and de novo Classification Request. Master Files are neither approved nor disapproved by the FDA, rather the FDA evaluates the information contained in Master Files during the review of applications that reference them.
In essence, Master Files streamline the regulatory process by enabling the sharing of critical information between manufacturers and the FDA while protecting sensitive commercial data. Master Files are not shared with users, only the FDA. In doing this, trade secrets are preserved while facilitating sound scientific evaluation. This FDA website provides information related to Device Master Files.
The FDA has five types of Master Files:
-
Device Master Files
-
Biologics Master Files
-
Drug Master Files (DMFs)
-
Food Master Files (FMFs)
-
Veterinary Medicine Master Files
These align closely to the different centers within the FDA, including the Center for Devices and Radiological Health (CDRH), Center for Biologics Evaluation and Research (CBER), Center for Drug Evaluation and Research (CDER), etc. Each center requires their own Master File if the information is to be referenced in a submission reviewed by that center.
Master Files can also be categorized by what information they contain. The FDA refers to these as functional types. Per the FDA, the following are the functional types of Master Files:
-
Facilities and manufacturing procedures and controls
-
Synthesis, formulation, purification, and specifications for chemicals, materials (e.g., an alloy, plastic, etc.), or subassemblies for a device
-
Packaging materials
-
Contract packaging and other manufacturing (e.g., sterilization)
-
Nonclinical study data
-
Clinical study data
The Device Master Files submitted by Formlabs are a combination of functional types, and include formulation and nonclinical study data. This is because we are sharing our confidential resin formulation with the FDA and providing nonclinical study data.
Common Terms
With respect to frequently used terms related to Master Files (MAFs), Formlabs is considered the MAF Holder as we are the organization or person filing an MAF. Any customer using BioMed materials in their submission and referencing our Master File(s) is the "applicant." Or, in the case they are submitting an Investigational Device Exemption (IDE), they would be called a "sponsor.” An "agent or representative for an MAF holder" is a person or organization authorized to represent the MAF holder before the FDA concerning the contents of the MAF. Many times this is utilized if the MAF holder is not based in the United States. In our case, Formlabs would take this role.
MAF Holder: The organization or person filing an MAF (for example, Formlabs)
Applicant: The organization or person filing a Premarket Approval (PMA) or a 510(k)
Sponsor: The organization or person filing an IDE (Investigational Device Exception)
Agent or Representative for an MAF Holder: The person or organization authorized to represent the MAF holder before the FDA concerning the contents of the MAF (for example, Formlabs or a Formlabs representative). This is normally for international MAF Holders.

Talk to Our Medical Sales Team
Whether you need to make patient-matched surgical tools or are prototyping for a cardiac medical device, we’re here to help. Formlabs Medical team are dedicated specialists who know exactly how to support you and your company's needs.
What Master Files Does Formlabs Have?
Formlabs has Device Master Files with the Center for Devices and Radiological Health (CDRH) for every BioMed resin we sell. This includes:
-
BioMed Clear Resin
-
BioMed Black Resin
-
BioMed White Resin
-
BioMed Durable Resin
-
BioMed Flex 80A Resin
-
BioMed Amber Resin
-
BioMed Elastic 50A Resin

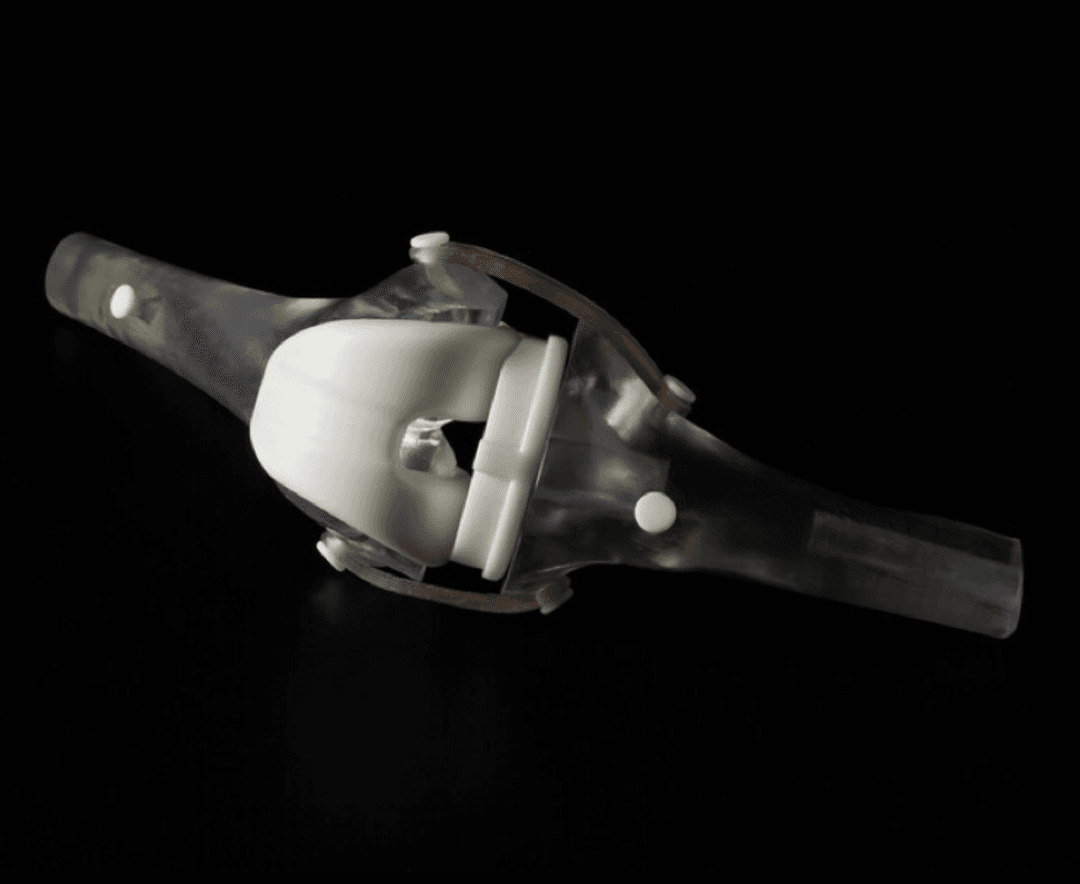
QuikBow® arms by Arbutus Medical printed in BioMed Black Resin on Form 4BL.
For each Master File submission, Formlabs provides the FDA with detailed information on the resin. This information includes the material description, the material formula, mechanical properties, and biocompatibility information, including full test reports for all endpoints. Given that this information is highly sensitive and confidential, we are not able to provide all this information directly to users. This is why we utilize the Master File process.

BioMed Resin Swatches
Each BioMed Resin swatch features embossed and debossed designs, 0.5-2.0 mm cutout thicknesses, as well as regulatory information unique to that resin.
Our Users’ Experience With Master Files
“When the FDA needed external validation, we were able to show that the external numbers exactly matched the Formlabs numbers and it solidified our case. Formlabs definitely helped expedite the process.”
Namratha Kumara, Product Quality and Regulatory Specialist, ImmersiveTouch
To date, Formlabs has written 20+ authorization letters to companies planning to submit a pre-market submission to the FDA. These have primarily been for 510(k) submissions, but there have also been some written for de novo submissions. Of those submissions, we know that at least six have received their clearances.

Learn how Adaptiiv uses Formlabs BioMed Resins to 3D print patient-specific bolus devices for radiation therapy.
During the review process, Formlabs is happy to help in any way we can, including answering any questions you may receive from the FDA regarding our materials. One question we have seen before is, “How do we know this data is actually relevant to your device?” With this, the FDA is basically evaluating if the Formlabs data referenced in the Master File is actually supporting your device, primarily when it comes to biocompatibility. One approach we have taken is to utilize surface area calculations to show that our data actually does support the product.
How To Get an Authorization Letter From Formlabs
If you plan to submit a pre-market submission to the FDA utilizing a Formlabs BioMed Resin, we can write an authorization letter for you to reference in your submission. To receive an authorization letter, email [email protected]. We will need the following information for an authorization letter:
-
Company Name
-
Company Address
-
Contact Name
-
Product Name
-
FDA Product Code
-
Submission Type
To learn more about BioMed Resins and Formlabs Master Files, watch the webinar or schedule a free Regulatory Affairs and Quality Assurance (RAQA) consultation.