
Formlabs Biocompatible Resins: A Comprehensive Guide To Choosing the Right Material
Formlabs currently offers more than 40 unique materials for stereolithography (SLA) 3D printing. This expansive library was designed to address a wide range of applications and functionalities across industries, but also includes a series of biocompatible resins created with healthcare applications in mind. These biocompatible materials cover a broad spectrum of mechanical properties and use cases. With so many options, it can be difficult to know where to start. This paper aims to help users compare and contrast our biocompatible offerings, and determine the best fit for their medical applications.
Formlabs Biocompatible Resins: A Comprehensive Guide To Choosing the Right Material

Formlabs currently offers more than 40 unique materials for stereolithography (SLA) 3D printing. This expansive library was designed to address a wide range of applications and functionalities across industries, but also includes a series of biocompatible resins created with healthcare applications in mind. These biocompatible materials cover a broad spectrum of mechanical properties and use cases. With so many options, it can be difficult to know where to start. This paper aims to help users compare and contrast our biocompatible offerings, and determine the best fit for their medical applications.
Material Overviews
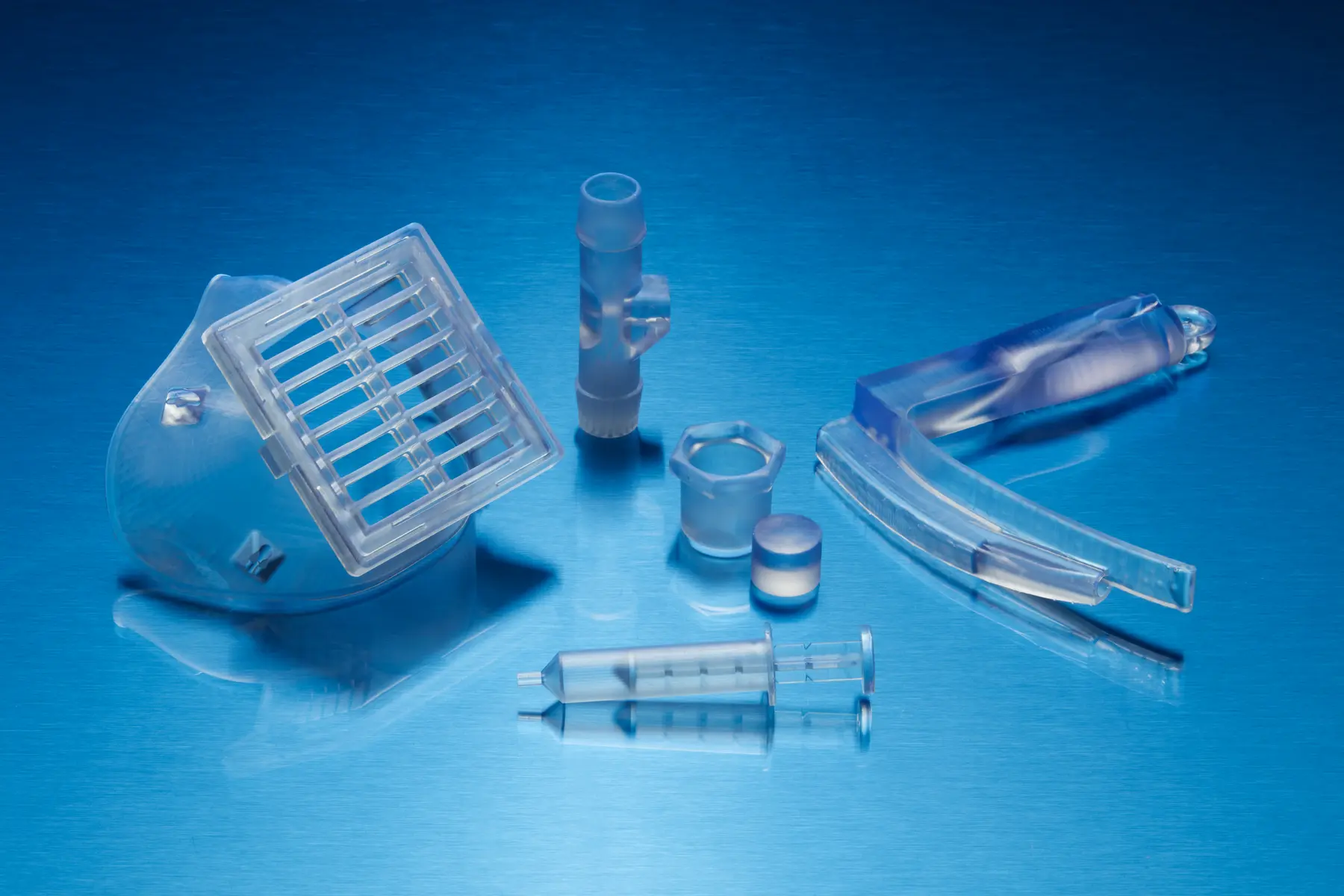
BioMed Clear Resin
BioMed Clear Resin is a transparent, hard, strong, and wear-resistant material for biocompatible applications requiring long-term contact with skin (>30 days), breathing gas pathways (>30 days), and mucosal membrane contact (>30 hours), or short-term contact with bone, tissue, and dentin (<24 hours).
Consider using BioMed Clear Resin for:
- End-use medical devices and device components requiring transparency
- Functional prototypes, molds, jigs, and fixtures
- Anatomical models for visualization and implant sizing
- Gas pathway connectors, adapters, and devices
- Silicone molds
- Bolus devices
Tough 1500 Resin
Tough 1500 Resin is a resilient material that offers similar strength and stiffness to polypropylene (PP). Parts made with Tough 1500 Resin are stiff, pliable, spring back quickly when bent, and are suited for applications requiring long-term skin contact (>30 days).
Consider using Tough 1500 Resin for:
- End-use medical devices and device components
- Functional prototypes, molds, jigs, and fixtures
- Orthoses and other wearable medical devices and components
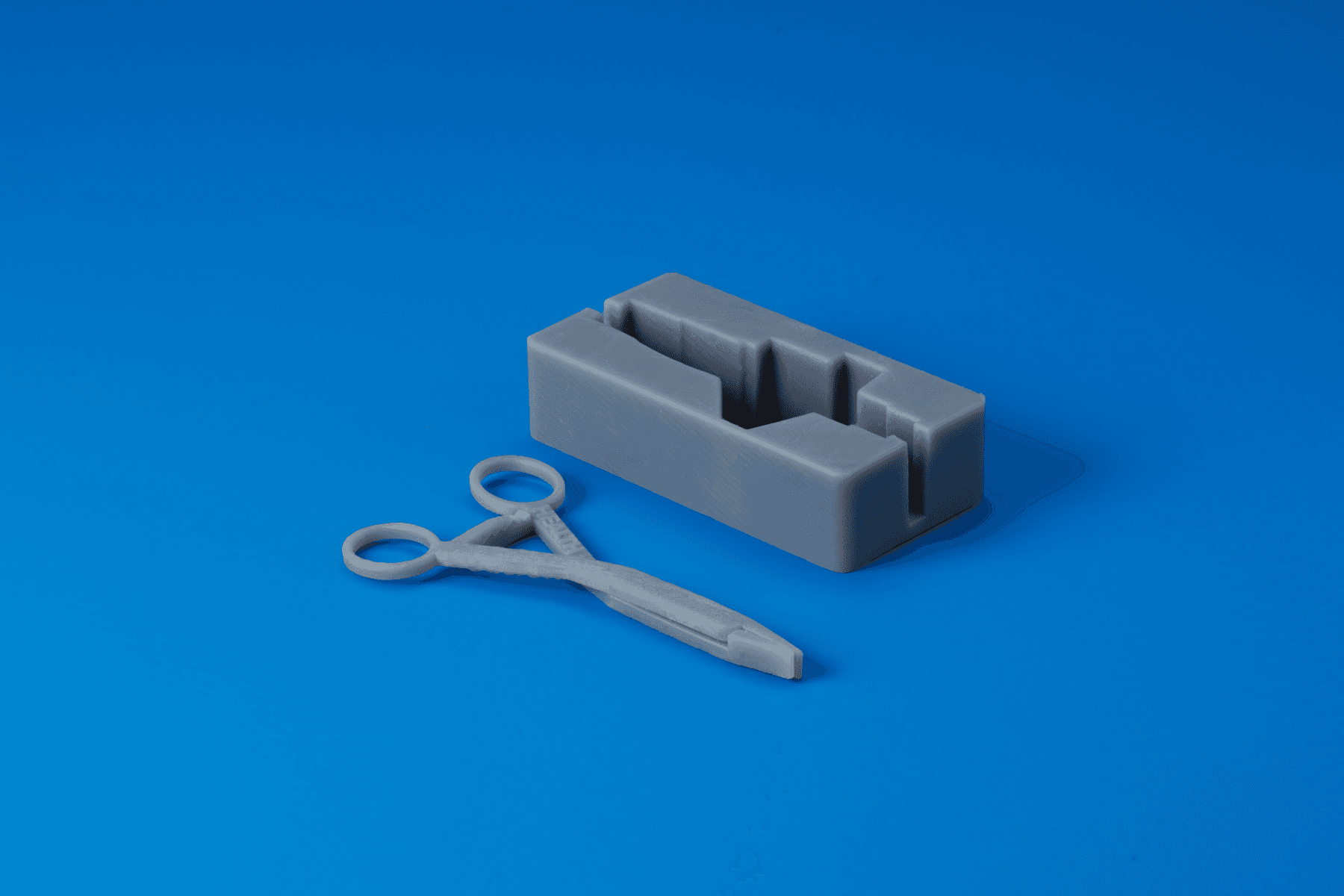
BioMed Durable Resin
BioMed Durable Resin is a transparent 3D printing material for biocompatible applications requiring impact, shatter, and abrasion resistance. This USP Class VI material can be used in applications for long-term contact with skin (>30 days) and mucosal membrane (>30 hours), or short-term contact with tissue, bone, and dentin (<24 hours).
Consider using BioMed Durable Resin for:
- Functional prototyping and limited run batches for human factors testing
- End-use medical devices requiring clarity and/or high impact resistance
- Patient-specific or single-use instruments • Cutting and drilling guides, surgical templates, sizers, and trials
- Bone simulation models for cutting and drilling
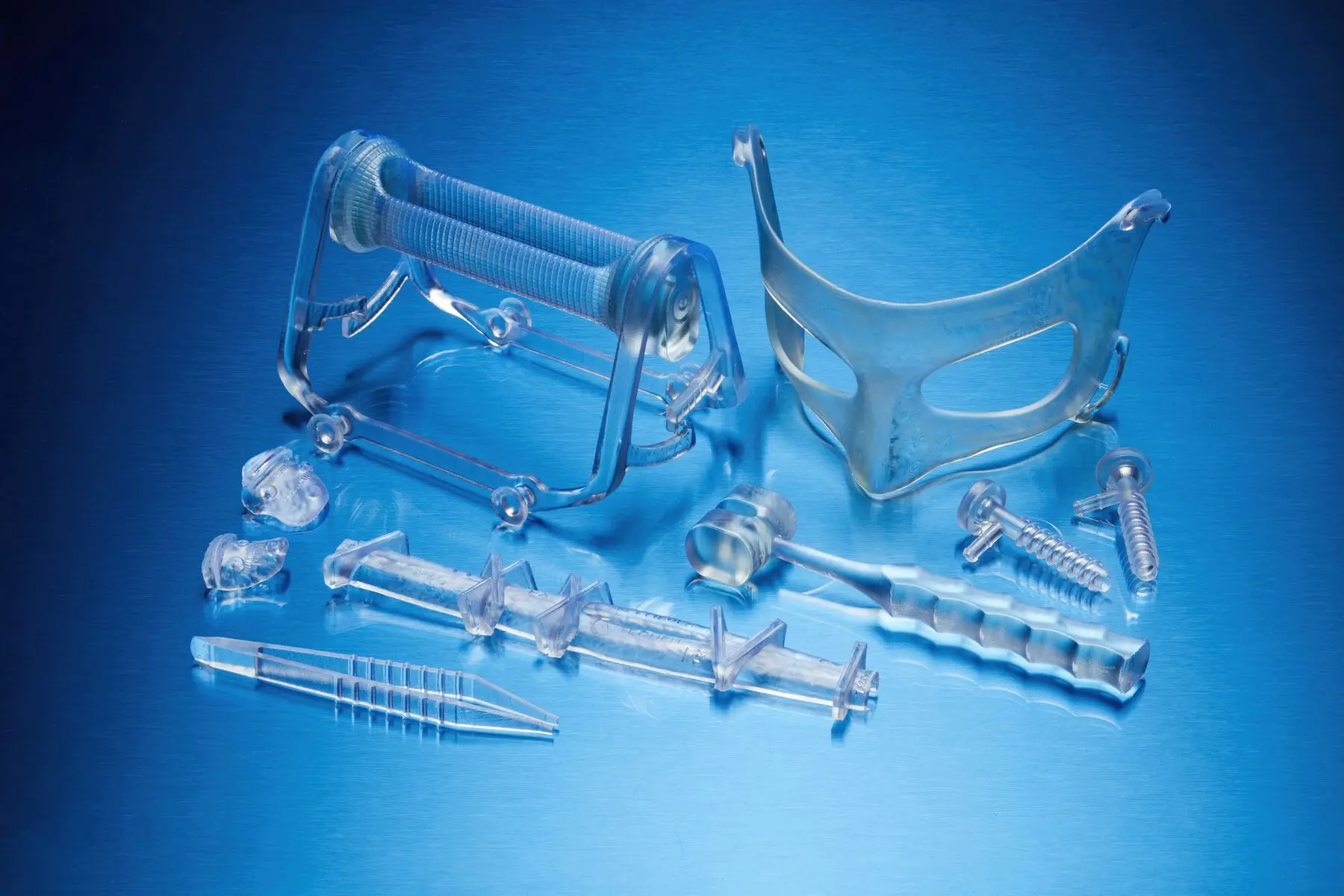
BioMed White Resin
BioMed White Resin is a rigid material for biocompatible applications requiring longterm skin contact (>30 days), or short-term bone, tissue, dentin, and mucosal membrane contact (<24 hours).
Consider using BioMed White Resin for:
- Functional prototypes, molds, jigs, and fixtures
- End-use medical devices and device components
- Patient-specific or single-use instruments
- Surgical templates, guides, trials, and sizers
- Anatomical models for surgical planning and practice
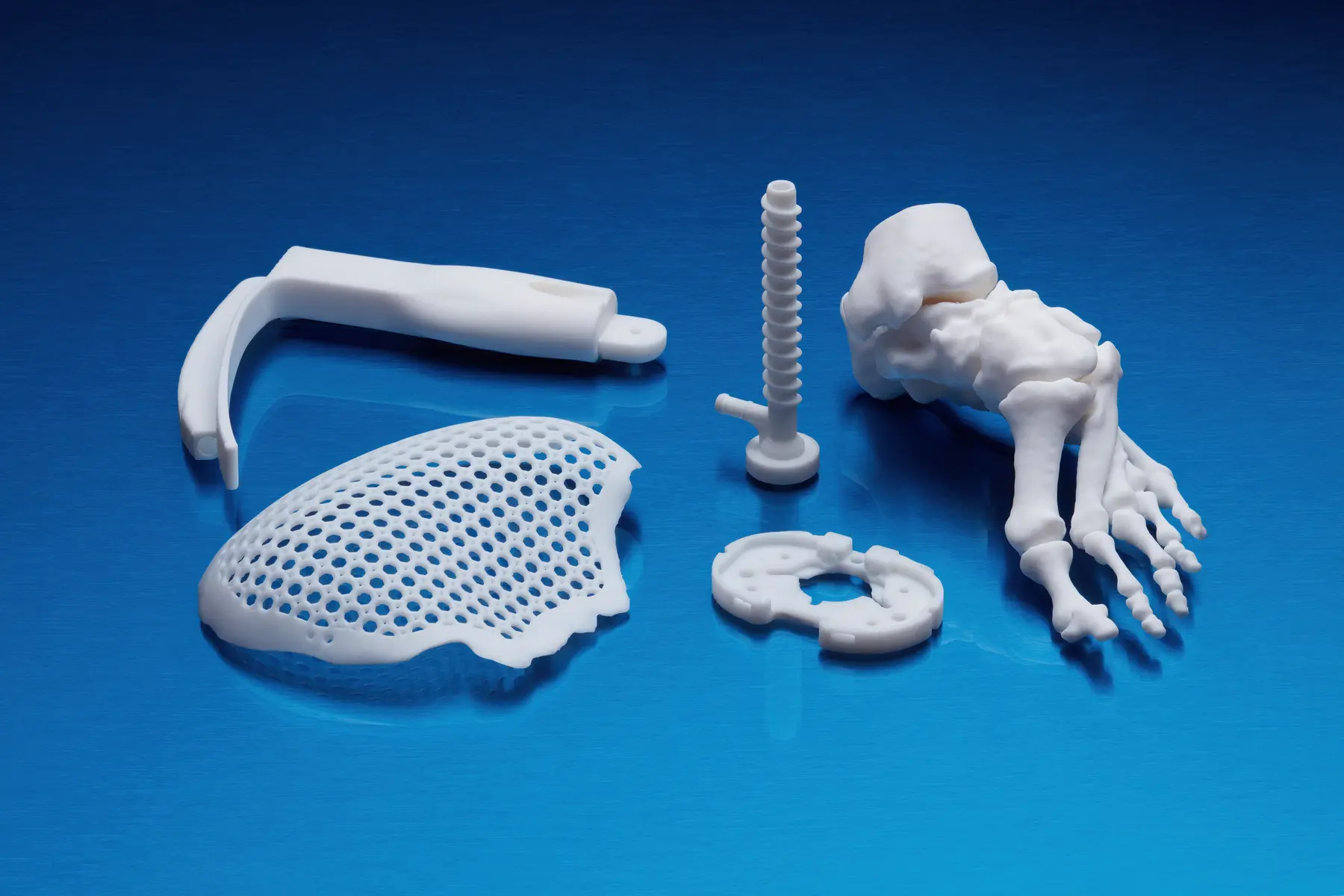
BioMed Black Resin
BioMed Black Resin is a rigid, matte material for biocompatible applications requiring long-term skin contact (>30 days), or short-term mucosal membrane contact (<24 hours).
Consider using BioMed Black Resin for:
- End-use medical devices and device components
- Functional prototypes, molds, jigs, and fixtures

BioMed Amber Resin
BioMed Amber Resin is a strong, stiff material for biocompatible applications requiring longterm skin contact (>30 days) or short-term bone, tissue, dentin, and mucosal membrane contact (<24 hours).
Consider using BioMed Amber Resin for:
- End-use medical devices and device components
- Cutting and drilling guides and templates
- Specimen collection kits
- Anatomical models for surgical planning

Silicone 40A Resin
Silicone 40A Resin is a 100% silicone, pliable, durable, and opaque elastomer for applications requiring long-term skin contact (>30 days) or limited mucosal membrane contact (≤24 hours).
Consider using Silicone 40A Resin for:
- Seals, gaskets, grommets, connectors, and dampeners for end-use medical devices
- Wearables, handles, or grippers
- Customized prosthetic components, orthoses, audiology models, and medical devices
- Flexible fixtures and soft molds
BioMed Elastic 50A Resin
BioMed Elastic 50A Resin is a soft, elastic, and transparent material for biocompatible applications requiring comfort and long-term skin contact (>30 days), or short-term mucosal membrane contact (<24 hours).
Consider using BioMed Elastic 50A Resin for:
- Comfortable end-use medical devices
- Soft medical device components
- Soft tissue models
BioMed Flex 80A Resin
BioMed Flex 80A Resin is a firm, flexible, and transparent material for biocompatible applications requiring durability and long-term skin contact (>30 days) or short-term mucosal membrane contact (<24 hours).
Consider using BioMed Flex 80A Resin for:
- Flexible medical devices and components
- Firm tissue models
No intended uses are provided for BioMed Resins. Here we offer representative examples of what customers have previously done with our products. Material properties may vary based on part design, manufacturing practices, and other methods. To instill confidence in potential users, Formlabs has tested biocompatibility and sterilization compatibility for common use cases and manufactures BioMed Resins in an ISO 13485 certified facility. Supporting documentation is readily available, please check out our RAQA webpage for more information.

Book a Consultation
Get in touch with our 3D printing experts for a 1:1 consultation to find the right solution for your business, receive ROI analyses, test prints, and more.
Mechanical Properties
The Formlabs material library offers resins with a wide range of mechanical properties, including both rigid and elastomeric biocompatible materials. Additional mechanical properties, and the most up-to-date values, can be found on each materials’ technical data sheet, which can be downloaded from the Formlabs store.
Rigid Mechanical Properties
| BIOMED CLEAR RESIN | TOUGH 1500 RESIN | BIOMED DURABLE RESIN | BIOMED WHITE RESIN | BIOMED BLACK RESIN | BIOMED AMBER RESIN | |
|---|---|---|---|---|---|---|
| Ultimate Tensile Strength (MPa) | 52 | 33 | 29 | 46 | 36 | 73 |
| Young’s Modulus (MPa) | 2080 | 1500 | 994 | 2020 | 1524 | 2900 |
| Elongation at Break (%) | 12 | 51 | 33 | 10 | 14 | 12 |
| Flexural Stress at 5% Strain (MPa) | 84 | 39 | 21 | 74 | 57 | 103 |
| Flexural Modulus (MPa) | 2300 | 1400 | 643 | 2020 | 1669 | 2500 |
| Shore Hardness | 78D | 76D | 75D | 80D | 77D | 67D |
| Notched IZOD (J/m) | 35 | 67 | 98 | 15 | 25 | 28 |
| Unnotched IZOD (J/m) | 449 | 1387 | 1340 | 269 | 348 | 142 |
| Heat Deflection Temp. at 1.8 MPa (°C) | 54 | 45 | 40 | 52 | 49 | 65 |
| Heat Deflection Temp. at 0.45 MPa (°C) | 67 | 52 | 46 | 67 | 68 | 78 |
| Coefficient of Thermal Expansion (μm/m/°C) | 82 | 97 | 103 | 90 | 107 | 66 |
For polyurethane parts that are suited for long-term contact with skin, you may be interested in checking out PU Rigid 1000 Resin or PU Rigid 650 Resin. PU Rigid 1000 Resin and PU Rigid 650 Resin require a longer workflow and additional equipment in order to get consistent, high-quality results and should be used in commercial or research facilities only. More information on using PU Rigid Resins can be found here.
Elastomer Mechanical Properties
| SILICONE 40A RESIN | BIOMED ELASTIC 50A RESIN | BIOMED FLEX 80A RESIN | |
|---|---|---|---|
| Ultimate Tensile Strength (MPa) | 5 | 2.3 | 7.2 |
| Stress at 100% Elongation (MPa) | 1 | 1.3 | 4.5 |
| Elongation at Break (%) | 230 | 150 | 135 |
| Tear Strength (kN/m) | 12 | 11 | 22 |
| Shore Hardness | 40A | 50A | 77-80A |
| Glass Transition Temperature (°C) | -107 | -36 | 37 |
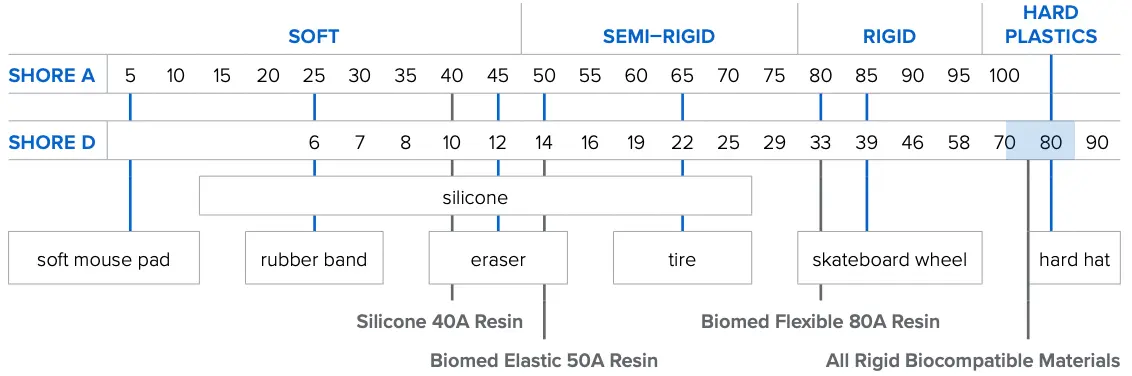
Durometers
A Shore Durometer value is used to indicate exactly how hard or soft a material is. Softer materials are measured on the Shore A scale, and harder materials on the Shore D scale. The table below shows how the durometer of our biocompatible materials compare to that of common household goods
Solvent Absorbency
Absorbancy of Formlabs materials was determined by measuring the weight gained after soaking a 1 cm cube in each solvent for 24 hours. These samples were printed and post-processed according to the standard manufacturing guidelines provided by Formlabs for each material. Biocompatibility of these materials was not tested after exposure, and therefore we cannot confirm that biocompatibility is maintained after biocompatible materials have been exposed to these solvents. Changes to mechanical and visual properties were also not tested and may or may not occur. This data is intended to help our users better understand the performance of our materials, but medical users who intend to expose our materials to harsh chemicals are encouraged to conduct their own testing.
| 24-HOUR WEIGHT GAIN (%) | BIOMED CLEAR RESIN | TOUGH 1500 RESIN | BIOMED DURABLE RESIN | BIOMED WHITE RESIN | BIOMED BLACK RESIN | BIOMED AMBER RESIN | SILICONE 40A RESIN | BIOMED ELASTIC 50 A RESIN | BIOMED FLEX 80A RESIN |
|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid 5% | 0.3 | 0.8 | 0.7 | 0.4 | 0.3 | 0.5 | < 0.1 | 1.5 | 1.42 |
| Acetone | 3.2 | 19 | 12.4 | 2.9 | 3.1 | 1.5 | 11.5 | 43.4 | 65.3 |
| Bleach ~5% NaOCl | 0.3 | 0.6 | 0.5 | 0.3 | 0.2 | 0.4 | < 0.1 | 0.6 | 0.5 |
| Butyl Acetate | 0.5 | 5 | 5 | 0.4 | 0.4 | < 0.1 | 92.3 | 133.1 | 97.5 |
| Diesel Fuel | 0.1 | 0.1 | 0.1 | < 0.1 | 0.1 | 0.2 | 32.9 | 7.9 | 5.1 |
| Diethyl Glycol Monomethyl Ether |
0.8 | 5.3 | 3 | 1 | 1 | 0.4 | 2.5 | 31.4 | 30.9 |
| Hydraulic Oil | < 0.1 | 0.2 | 0.02 | < 0.1 | 0.2 | 0.4 | 10 | 3.9 | 2.5 |
| Hydrogen Peroxide (3%) | 0.3 | 0.7 | 0.6 | 0.3 | 0.3 | 0.6 | < 0.1 | 0.9 | 0.7 |
| Isooctane (gasoline) | < 0.1 | < 0.1 | 0.02 | < 0.1 | < 0.1 | < 0.1 | 69.8 | 15.6 | 9 |
| Isopropyl Alcohol | < 0.1 | 3.2 | 2 | 0.2 | 0.2 | < 0.1 | 5.9 | 39.2 | 25.9 |
| Mineral Oil (light) | 0.2 | < 0.1 | 0.1 | < 0.1 | 0.2 | 0.2 | 2 | 0.7 | 0.4 |
| Mineral Oil (heavy) | 0.2 | < 0.1 | 0.1 | < 0.1 | 0.2 | 0.2 | 1.6 | 0.4 | 0.2 |
| Salt Water (3.5% NaCl) | 0.3 | 0.7 | 0.5 | 0.4 | 0.3 | 0.5 | < 0.1 | 0.6 | 0.5 |
| Skydrol 5 | 0.5 | 0.5 | 0.5 | 0.6 | 0.3 | 23.2 | 41.2 | 28.1 | |
| Sodium Hydroxide Solution (0.025% PH 10) |
0.3 | 0.7 | 0.5 | 0.3 | 0.3 | 0.5 | < 0.1 | 0.7 | 0.6 |
| Strong Acid (HCl conc) | 0.1 | 4.4 | 0.7 | 0.2 | 0.2 | 0.7 | 0.4 | 45.6 | 37.3 |
| Tripropylene Glycol Methyl Ether (TPM) |
0.4 | 0.6 | 1.1 | 0.6 | 0.6 | 0.2 | 43.6 | 31.2 | |
| Water | 0.3 | 0.7 | 0.5 | 0.3 | 0.3 | 0.5 | < 0.1 | 0.7 | 0.6 |
| Xylene | 0.3 | 3.2 | 4.8 | 0.3 | 0.3 | 0.1 | 163.9 | 112.5 |

Get Started with 3D Printing
Formlabs' complete, easy-to-use ecosystem makes it simple to get started with 3D printing. Explore our 3D printers and materials to find the right fit for your needs.
Resolution and Print Time
Recommended layer thicknesses vary between 50 μm and 100 μm for biocompatible materials. A larger layer thickness should be used when speed is a priority, and a smaller layer thickness should be used when accuracy is a priority. Rigid materials tend to be able to achieve greater accuracy than elastomers when printing with a lower layer thickness.
| LAYER THICKNESS | FORM 3B/3BL PRINT TIME (4X 5 CM CUBES) | FORM 4B PRINT TIME (4X 5 CM CUBES) | |
|---|---|---|---|
| BioMed Clear Resin | 100 μm | 8 h 18 min | 4 h 1 min |
| 50 μm | 13 h 48 min | 10 h 49 min | |
| Tough 1500 Resin | 100 μm | 7 h 33 min | 3 h 40 min |
| 50 μm | 14 h 47 min | 11 h 29 min | |
| BioMed Durable Resin | 100 μm | 6 h 38 min | 4 h 33 min |
| BioMed White Resin | 100 μm | 7 h 13 min | 3 h 48 min |
| 50 μm | 13 h 31 min | ||
| BioMed Black Resin | 100 μm | 11 h 23 min | 4 h 38 min |
| 50 μm | 16 h 42 min | 12 h 37 min | |
| BioMed Amber Resin | 100 μm | 16 h 37 min | 2 h 56 min |
| 50 μm | 24 h 50 min | 10 h 13 min | |
| Silicone 40A Resin | 100 μm | 16 h 56 min | |
| BioMed Elastic 50A Resin | 100 μm | 9 h 23 min | 6 h 48 min |
| BioMed Flex 80A Resin | 100 μm | 14 h 41 min | 7 h 26 min |
For rapid prototyping or anatomical modeling applications when speed is a priority and biocompatibility/sterilizability is not, you may want to consider Fast Model Resin or Draft Resin.
Visual Properties
Color and Opacity
BioMed Clear Resin
Translucent, slight purple tint

Tough 1500 Resin
Opaque gray

BioMed Durable Resin
Translucent, slight blue tint

BioMed White Resin
Opaque white

BioMed Black Resin
Opaque black

BioMed Amber Resin
Semi-transparent, amber tint

Silicone 40A Resin
Opaque, dark gray
BioMed Elastic 50A Resin
Semi-transparent, slight yellow tint

BioMed Flex 80A Resin
Semi-transparent, slight blue tint

Radiodensity
Radiodensity, sometimes referred to as radiopacity, is the measure of how effectively a material blocks or attenuates radiation. These values can be useful when designing bolus devices, imaging phantoms, or other parts intended for radiation.
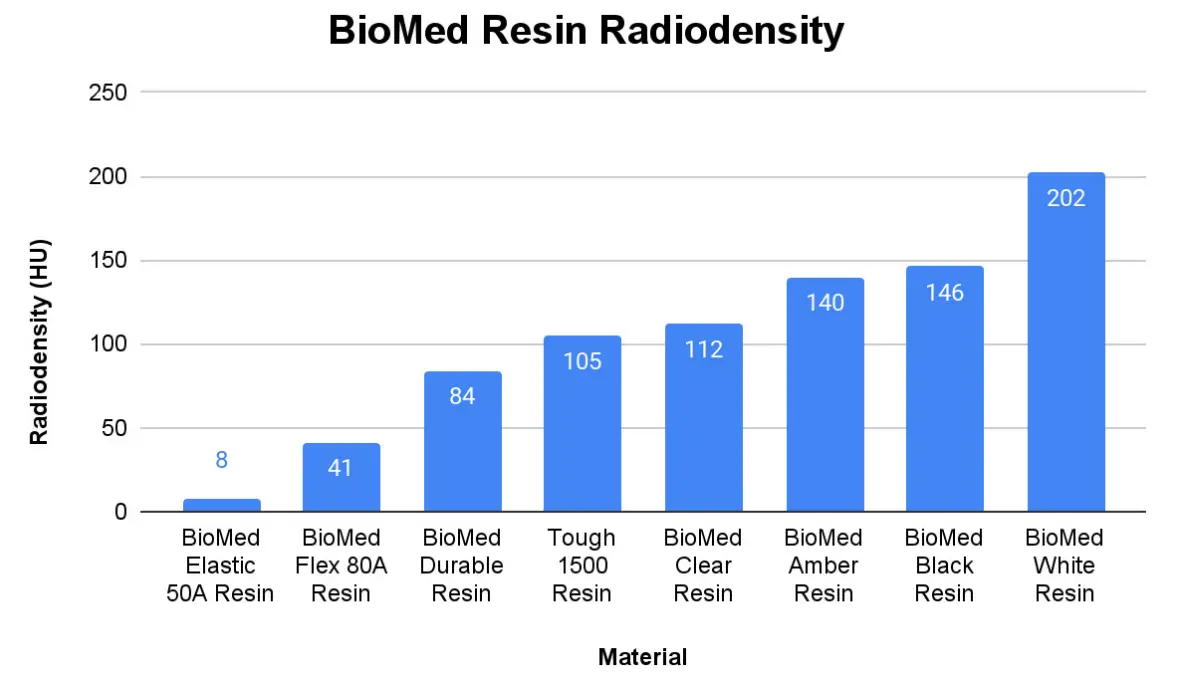
Formlabs offers some engineering and dental materials with higher and lower radiopacities than BioMed materials. Values for some of these materials are featured in the table below. To achieve radiodensities even lower than those featured here, you may want to consider looking into selective laser sintering (SLS) 3D printing.
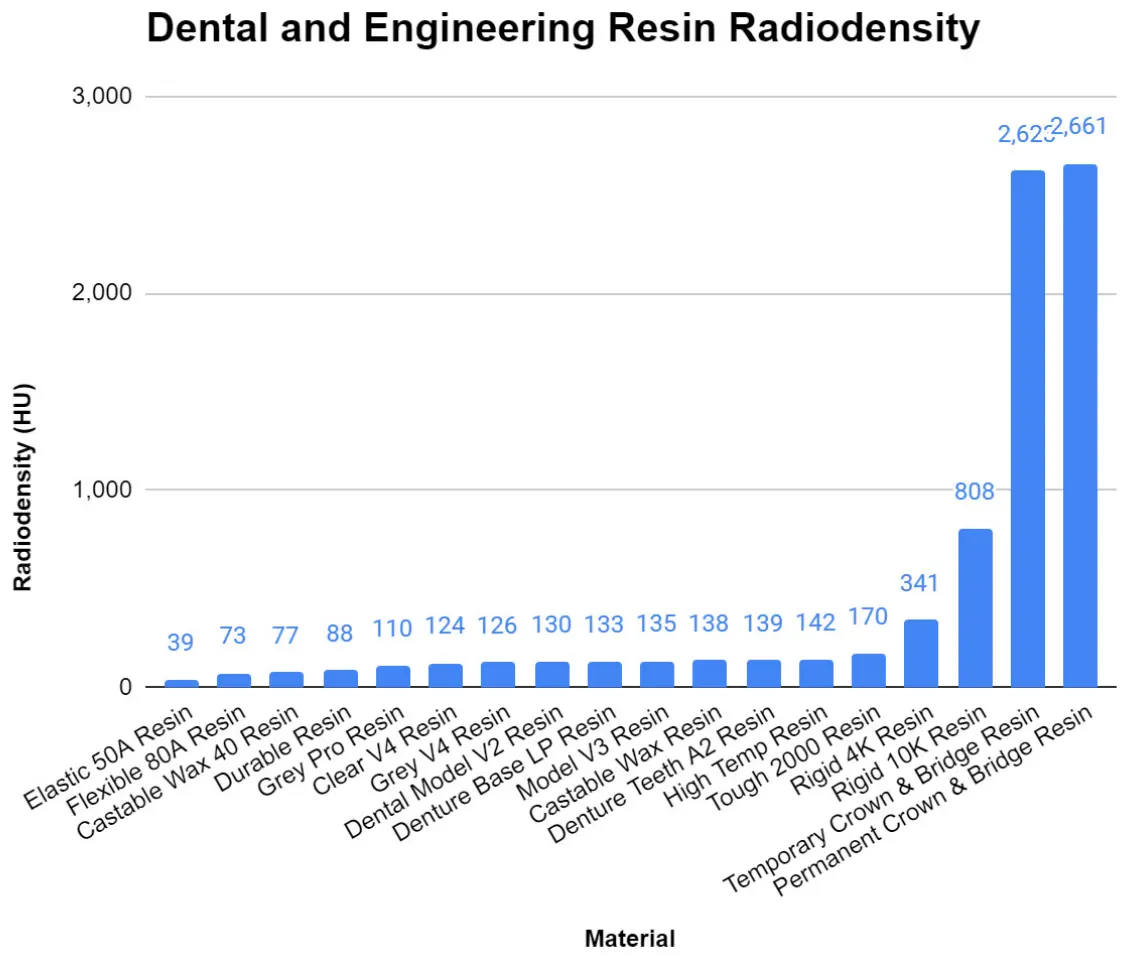
You can learn more about the methods used to determine these values in this paper, which was jointly published by St. Louis Children’s Hospital, St. Louis University School of Medicine, and the Washington University School of Medicine.
Certifications and Standards
Biocompatibility and Sterilization
The contact types and durations outlined in the table below indicate the conditions for which the materials were tested. These materials may also perform favorably under additional conditions, but testing for additional contact types and durations must be conducted by the user. Sterilization guidelines and results for sterilizable materials are available upon request, you can get in contact with a medical 3D printing expert here.
| CONTACT TYPE | SKIN | MUCOSAL MEMBRANE | BONE, TISSUE, & DENTIN | PHARMACEUTICAL CONTAINERS, DRUG DELIVERY & MEDICAL DEVICE COMPONENTS |
MUCOSAL MEMBRANE | BREATHING GAS PATHWAYS | |
|---|---|---|---|---|---|---|---|
| Contact Duration | > 30 days | < 24 hours | < 24 hours | >30 hours | >30 hours | ||
| Application ISO Standard |
EN ISO 10993-EN ISO 10993 EN ISO 10993-5 |
EN ISO 10993-1 EN ISO 10993-3 EN ISO 10993-5 |
USP Pyrogen | USP Class VI |
EN ISO 10993-1 EN ISO 10993-3 EN ISO 10993-5 EN ISO 10993-10 EN ISO 10993-11 EN ISO 10993-23 |
EN ISO 18562-1 EN ISO 18562-2 EN ISO 18562-3 EN ISO 18562-4 |
Sterilization Compatibility |
| BioMed Clear Resin | X | X | X | X | X | X | Steam, Gamma, Et0,E-Beam |
| BioMed Durable Resin | X | X | X | X | X | Steam, other methods in progress | |
| BioMed White Resin | X | X | X | X | Steam, Gamma, Et0,E-Beam | ||
| BioMed Amber Resin | X | X | X | Steam, Gamma, Et0,E-Beam | |||
| BioMed Black Resin | X | X | X | Steam, Gamma, Et0,E-Beam | |||
| BioMed Flex 80A Resin | X | X | In progress | ||||
| BioMed Elastic 50A Resin | X | X | Disinfection | ||||
| Tough 1500 Resin | X | Steam, Gamma, EtO, E-Beam | |||||
| Silicone 40A Resin | X | X | Not tested |
FDA Master Files
Formlabs has Device Master Files (MAF) with the FDA for BioMed Black Resin, BioMed Clear Resin, and BioMed Durable Resin. Information in the MAF may be referenced in your Premarket Approval Application, PreMarket Notification (510(k)), Investigational Device Exemption Application, or other submissions to the FDA. Please contact your sales representative for a letter of authorization.
User Manuals and Documentation
User Experiences
Patient-Specific Bolus Devices by Adaptiiv Using BioMed Clear Resin
Adaptiiv is a Nova Scotia-based company that specializes in 3D printed medical devices and software development for cancer treatment. Their regulatory-cleared software is utilized by radiologists to design patient-specific bolus devices for intracavitary and interstitial radiation therapy. These devices are then printed by Adaptiiv using BioMed Clear Resin, and shipped to the treatment provider using their Adaptiiv On Demand service.
Traditionally, bolus devices are made by radiation therapists using dental wax or a rubbery sheet called Superflab. These materials are difficult to manipulate and don’t always conform accurately to patient anatomy. 3D printing patient-specific bolus devices with BioMed Clear Resin allows for geometry that perfectly contours patient anatomy, resulting in a more accurate and timely delivery of radiation to the targeted site.
Learn more about Adaptiiv’s use of the Formlabs ecosystem for bolus production here.

Intracavitary and interstitial bolus devices created by Adaptiiv and printed using BioMed Clear Resin.
TechFit Digital Surgery Achieves FDA Clearance With BioMed Clear Resin
TechFit Digital Surgery is a small medical device company that streamlines the surgical planning workflow using digital planning, 3D printing, and patient-specific implants. Techfit achieved FDA clearance for their digital orthognathic surgery system, Digitally Integrated Surgical Reconstruction Platform (DISRP®), in a record six months with the help of Formlabs’ FDA Master File for BioMed Clear Resin, and insight from Formlabs’ Regulatory Affairs and Quality Assurance (RAQA) team.
An FDA Master File is a submission to the FDA that contains detailed, confidential information about a product, process, or material used in the manufacturing of a medical device. The BioMed Clear Resin Master File includes the manufacturing process, extensive documentation, and testing data. By leveraging the Master File, TechFit was able to avoid duplicating costly chemical characterization tests. This saved them an estimated $70,000-$80,000 and significantly accelerated their FDA clearance timeline. The Master File, combined with the support of the Formlabs RAQA team, allowed TechFit to navigate the complex regulatory process efficiently and bring their innovative surgical solution to market in the US quickly.
Learn more about TechFit Digital Surgery’s path to FDA clearance using BioMed Clear Resin and the Formlabs RAQA team here.
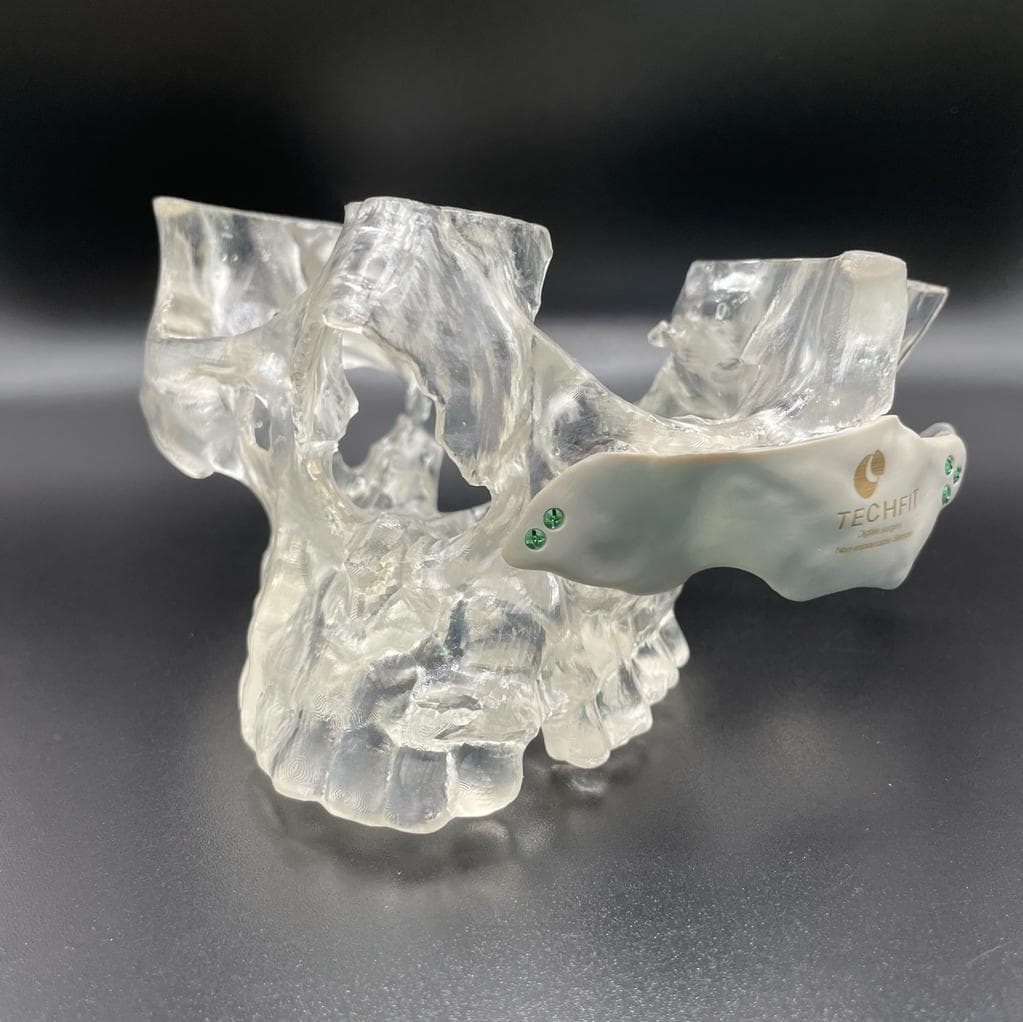

Anatomical models printed in BioMed Clear Resin by Techfit.
Custom Cutting and Drilling Guides at Trabtech With Biomed Durable Resin
Trabtech is a medical technology development firm that specializes in designing and producing custom-made cutting and drilling guides using 3D printing. In 2022, they conducted a doctoral research project centered around the development of a vertebral anchor system. This study was designed by Dr. Turan Najafov, one of the distinguished doctors in the Department of Orthopedics and Traumatology at Maltepe University. Their objective was to compare the pullout strength of Dr. Najafov’s newly designed and 3D printed system, referred to as the angled transpedicular screw anchor system (ATSAS), with the conventional 6.5 mm pedicle screws (PS) in a control group. The results of the study revealed that the pullout strength of the ATSAS was remarkably superior, boasting a 35% increase compared to the traditional PS.
Learning of the development of BioMed Durable Resin, Trabtech immediately saw the potential of 3D printing their system using a strong, biocompatible material. Dr. Turan Najafov, from the Department of Orthopedics and Traumatology at Maltepe University, in partnership with Trabtech, stated that “The introduction of BioMed Durable Resin represents a significant milestone in the evolution of surgical guides. Its exceptional mechanical properties, combined with the versatility of 3D printing, have empowered Trabtech to develop tools that enhance surgical precision and efficiency. As we continue to explore the potential of this remarkable material, we envision its applicability in diverse regions for cutting guides and surgical instruments that are currently undergoing trials. Our collaboration with Formlabs and the integration of this exceptional material into our designs have opened new possibilities in the field of medical technology.”
Learn more about Trabtech’s experience with BioMed Durable Resin here.



Cutting and drilling guides printed by Trabtech in BioMed Durable Resin.
Researchers Use BioMed White Resin for Custom Prosthetic
During a routine check-up at ZooTampa, caretakers discovered a life-threatening cancerous lesion on Crescent, a 25-year-old great hornbill, at the base of its casque (the yellow structure on the top of the head). The tumor would have to be removed, but because of its proximity to the bird’s sinuses, a prosthesis would be required to replace the function of the missing portion of the casque.
Researchers from the University of South Florida Morsani College of Medicine’s Department of Radiology designed and printed a custom prosthetic for Crescent using BioMed White Resin. Hours after surgery, Crescent began preening. BioMed White Resin happened to be compatible with the yellow preening oils produced by the grand hornbill, and, before long, the prosthesis had become the same shade of yellow as the previous casque.
Learn more about this great hornbill’s beak with a BioMed White Resin prosthetic here.

Great hornbill fitted with a BioMed White Resin prosthetic casque.
Rapid Production of COVID-19 Testing Swabs With BioMed Amber Resin
When the COVID-19 pandemic first hit, there was an acute shortage of test swabs. Teams from USF Health, Northwell Health, Tampa General Hospital, and Formlabs worked together to develop a 3D printed swab using BioMed Amber Resin.
After establishing a design and printing process that worked, the design was shared with hospitals, health systems, the U.S. military, and clinics around the globe. The USF-patented design for the 3D printed nasopharyngeal swab was shared free of charge to help accelerate adoption and, to date, has been printed over 100 million times in countries all over the world. In 2023, the United States Patent and Trademark Office awarded the project a Patents for Humanity Award, which “recognizes innovators who use game-changing technology to meet global humanitarian challenges.”
Learn more about rapid production of COVID-19 testing swabs here.

COVID-19 testing swabs printed using BioMed Amber Resin.
3D Printing Water-Tight Gaskets With Silicone 40A Resin
FINIS, Inc. is a California-based company that designs innovative swimwear and accessories for swimmers of all skill levels. David Beittel, a senior designer at FINIS, Inc., was originally prototyping with traditional, labor-intensive silicone moldings and fused deposition modeling (FDM) 3D printing before transitioning to Form 3+ and Silicone 40A Resin. Printing with Silicone 40A Resin has allowed FINIS, Inc. to create more detailed and isotropic parts than previously possible, and achieve characteristics close to final production materials without the long lead times associated with traditional manufacturing.
The 3D printed silicone gaskets pictured below successfully underwent rigorous water tightness assessments within a swimming pool environment and demonstrated elasticity closely mirroring final production characteristics. Silicone 40A Resin has become an essential tool for refining designs and ensuring optimal performance throughout FINIS, Inc.’s development process.
Learn more about FINIS, Inc.’s prototyping process using SIlicone 40A Resin here.

FINIS, Inc. goggles prototype using Silicone 40A Resin.
Leveraging BioMed Elastic 50A Resin at Point of Care
Dr. Prashanth Ravi, an Assistant Professor in the Department of Radiology at the University of Cincinnati, has utilized Formlabs’ array of biocompatible resins extensively over the years. Dr. Ravi identified an application for the material: an anatomical model used for device sizing right in the operating room. He states, “One clear application is in device sizing for left atrial appendage closure planning to treat atrial fibrillation. If the cardiologist wants to take the sterilized 3D printed anatomic model into the operating room to actually size the device and communicate with the surgical team prior to the intervention, BioMed Elastic 50A Resin is the material we would be using.”
At Baystate Health, Greg Gagnon is a specialist in 3D printing and is responsible for preparing 3D printed bolus devices and other medical tools for physicians. It wasn’t until the launch of BioMed Elastic 50A Resin that he was able to utilize direct 3D printing of elastomeric material in his workflow. He says that he “really enjoyed the material. Relative electron density was almost identical to water which is 1.0 g/cm3 and is great for 3D printing patient boluses. [We] already have some designs completed to start testing and expand our use cases into our surgical field.”
You can read more on Dr. Prashanth Ravi and Greg Gagnon’s thoughts on BioMed Elastic 50A Resin here.

Bolus device printed using BioMed Elastic 50A Resin.
Do you have questions about using SLA printing for biocompatible part production? Set up a meeting with a Formlabs expert who can answer your questions. Request a free sample part to see Formlabs 3D printed materials firsthand.
Request a free sample part to see Formlabs 3D printed materials firsthand and contact our 3D printing specialist to find the right solution for your application.