3D Printed Adapters Snorkel Mask Conversion to Emergency PPE
What are Conversion Kits?
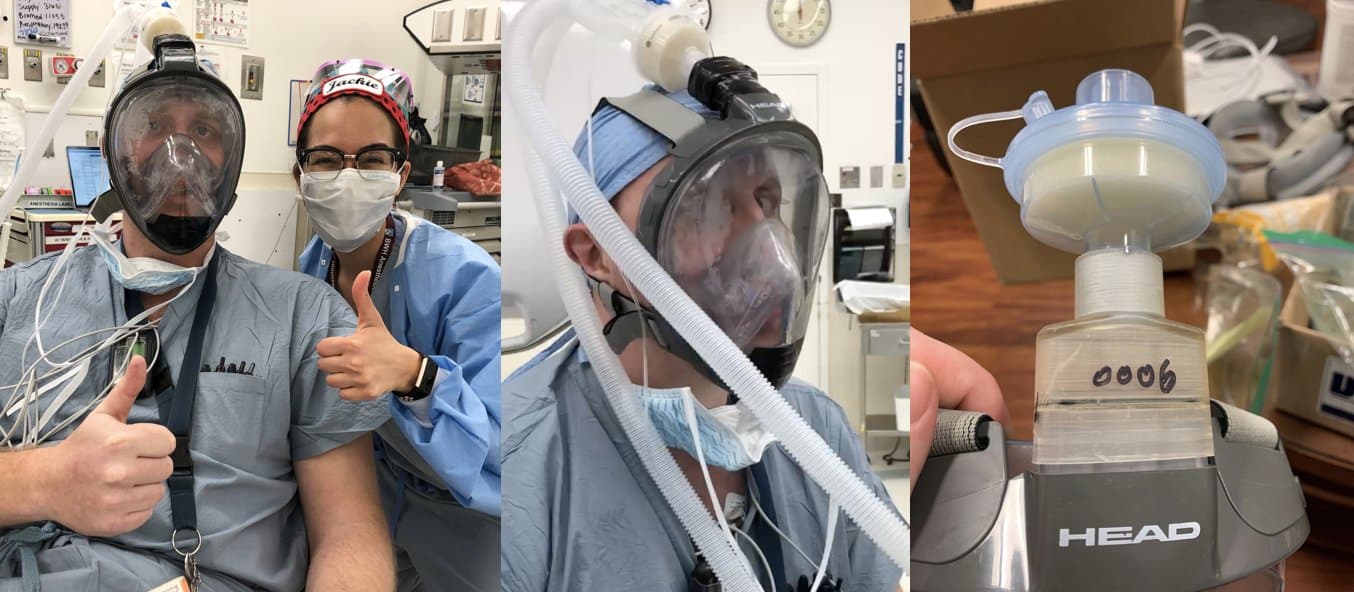
Dr. Alex Stone and Dr. Jacqueline Boehme from a leading hospital in Boston
Problem
Front-line providers are running out of personal protective equipment (PPE). At many hospitals, there is a very limited supply of PPE remaining. PPE includes face masks and/or respirators (e.g. N95 masks) and face shields. In addition, the typical forms of PPE are disposable and more durable solutions are needed given the limited supply. Moreover, most PPE is designed to be used for minutes at a time, not for an entire day.
Solution
Adapting a full face snorkel mask to fit a filter already in use for breathing circuits and in hospitals. Because this filter is reusable, and unavailable/unknown to the general public, it will be less at risk to be taken from providers treating ill patients.
Validation Status
Dr. Alex Stone and Dr. Jacqueline Boehme from a leading Boston hospital are currently testing and validating designs for: seal, breathability, fogging, and ability to communicate with other physicians while wearing it.
This page describes efforts originating in the US. Find out more about the development of mask adapters in Spain.
Disclaimer
Formlabs has created this website and has become involved in varying degrees with the projects described here during a global medical crisis. Formlabs is working on many projects to address global healthcare-related supply shortages around the world, but in trying to mitigate these shortages, patient safety is still Formlabs’ top concern. We must remind those who are helping to alleviate these shortages that masks, swabs, face shields, and other 3D printed products intended to prevent or treat COVID-19 are medical devices. These devices must be safe for their intended purpose and anyone considering the manufacturing of these products should consider the following items:
-
Formlabs is a manufacturer of 3D printing materials capable of fabricating finished devices according to their labeling and any other product manufactured from these materials should be verified and validated according to their intended purpose.
-
You may be fabricating a device that does not have the required regulatory approvals and clearances. If you are fabricating devices, follow the guidelines on the label for each material. You may seek to obtain reliable regulatory advice.
-
Please consider local regulations, material safety data sheets, software capabilities, sterilization requirements, and institutional requirements before 3D printing medical devices.
-
Regulatory agencies (such as the FDA) may consider expedited review of manufacturing information and/or premarket submissions.
Formlabs cannot warrant that any products not manufactured by Formlabs are suitable for their intended purpose.
Regulatory Statement
NP swabs, intended to collect specimens from a patient, are Class I devices exempt from premarket notifications according to 21 CFR § 880.6025 Absorbent tipped applicator.
FDA requires medical device manufacturers to register their facility and list their products according to 21 CFR § 807.20.
Formlabs facilities are registered:
Formlabs Inc. Registration Number: 3010279788 (Link)
Formlabs Ohio Inc. Registration Number: 3015491441 (Link)
Formlabs’ 3D Printed NP Swabs are listed. (Link)