Leverage Our Expertise for Your Quality & Regulatory Needs
In-House Regulatory, Quality, and Medical Device Manufacturing Expertise
Formlabs Medical Device Standards, Statements, and Certifications


The Ultimate Guide to Quality Assurance and Regulatory Affairs in Medical 3D Printing

Formlabs Guide to SLA Quality Control Management for Healthcare Innovators
Calibration Documentation, FDA Master Files, and More

See Also

What Is Biocompatibility and What Should It Mean to You? Hear From Nelson Labs


Formlabs x TÜV SÜD: Exploring Additive Manufacturing and Regulatory Requirements in the EU
Cleared, Commercialized Devices

See Also
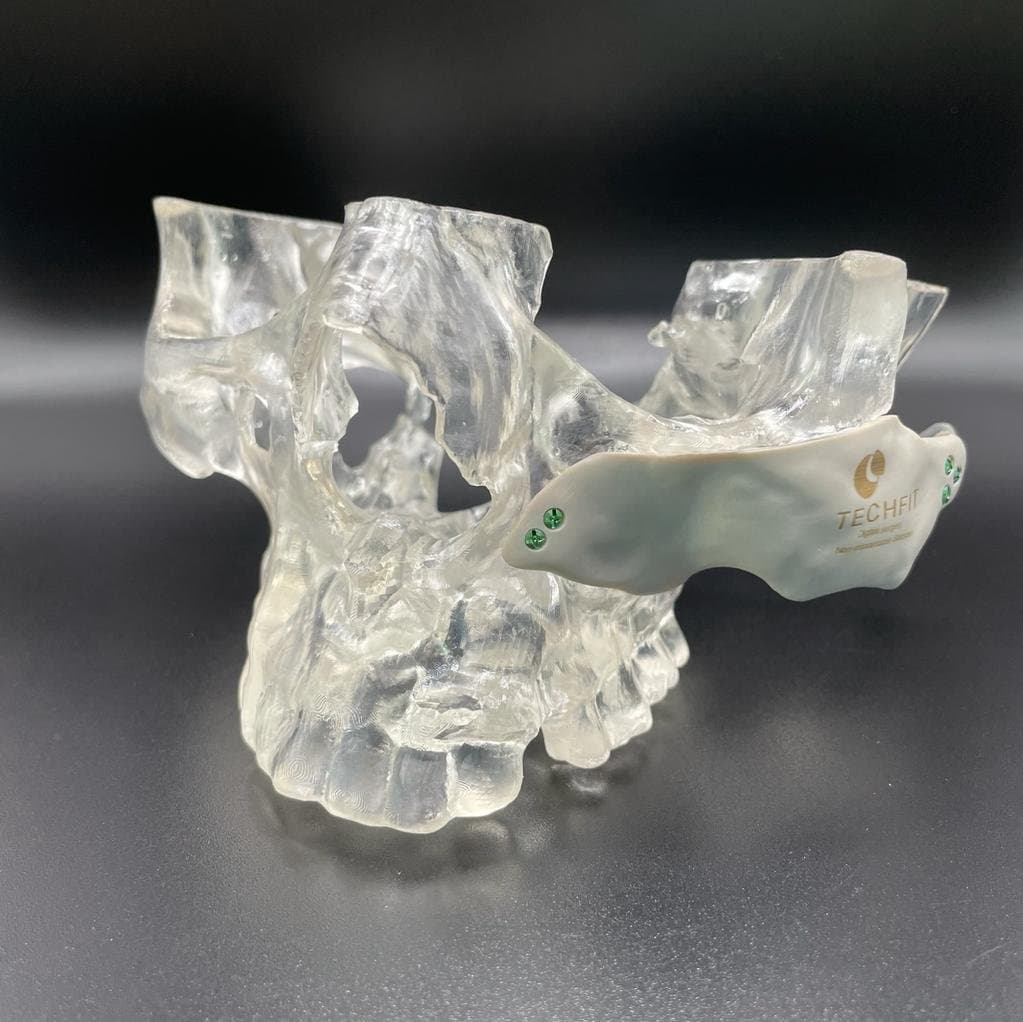
Techfit Achieves Fast, Under-Budget FDA Clearance With Help From Formlabs RAQA Team


A Full Suite of 3D Printing Solutions

Form 4B
Parts requiring biocompatibility and sterilization
Medical device prototypes, jigs, fixtures, molds, and end-use parts
Visual aids for diagnosis and education
Models for diagnostic use in FDA-cleared workflows

Form 4BL
Mid-size to large medical device prototypes
Molds and dies
Surgeon-specific or patient-specific objects
Full-size anatomical models for adult anatomy

Fuse 1+ 30W
Functional medical device prototypes
Complex medical parts printed without supports
End use parts: enclosures, connectors, manifolds, prosthetics, insoles
Long-lasting, durable jigs and fixtures
The Best Selection of Advanced Medical 3D Printing Materials
Choose between our wide range of proprietary materials, certified third-party materials from top-tier manufacturers, or experiment with any unvalidated 405nm photopolymer resin with Open Material License. Our library comprises over 45 materials, including a variety of biocompatible formulations and high-precision, durable materials for medical device manufacturing to fuel innovation in 3D printing medical applications. Formlabs biomedical and medical device resins are designed and manufactured within our robust Quality Management System that is ISO 13485 and EU MDR certified in our certified FDA Registered facility.


Upgrade Your Medical Device Manufacturing Today
Explore our 3D printers or contact a 3D printing expert today to find the solution to meet your business goals.