Innovative, Low-Cost Inhalers Enable Access for Millions: 3D Printing Powers Lean R&D for Medical Devices
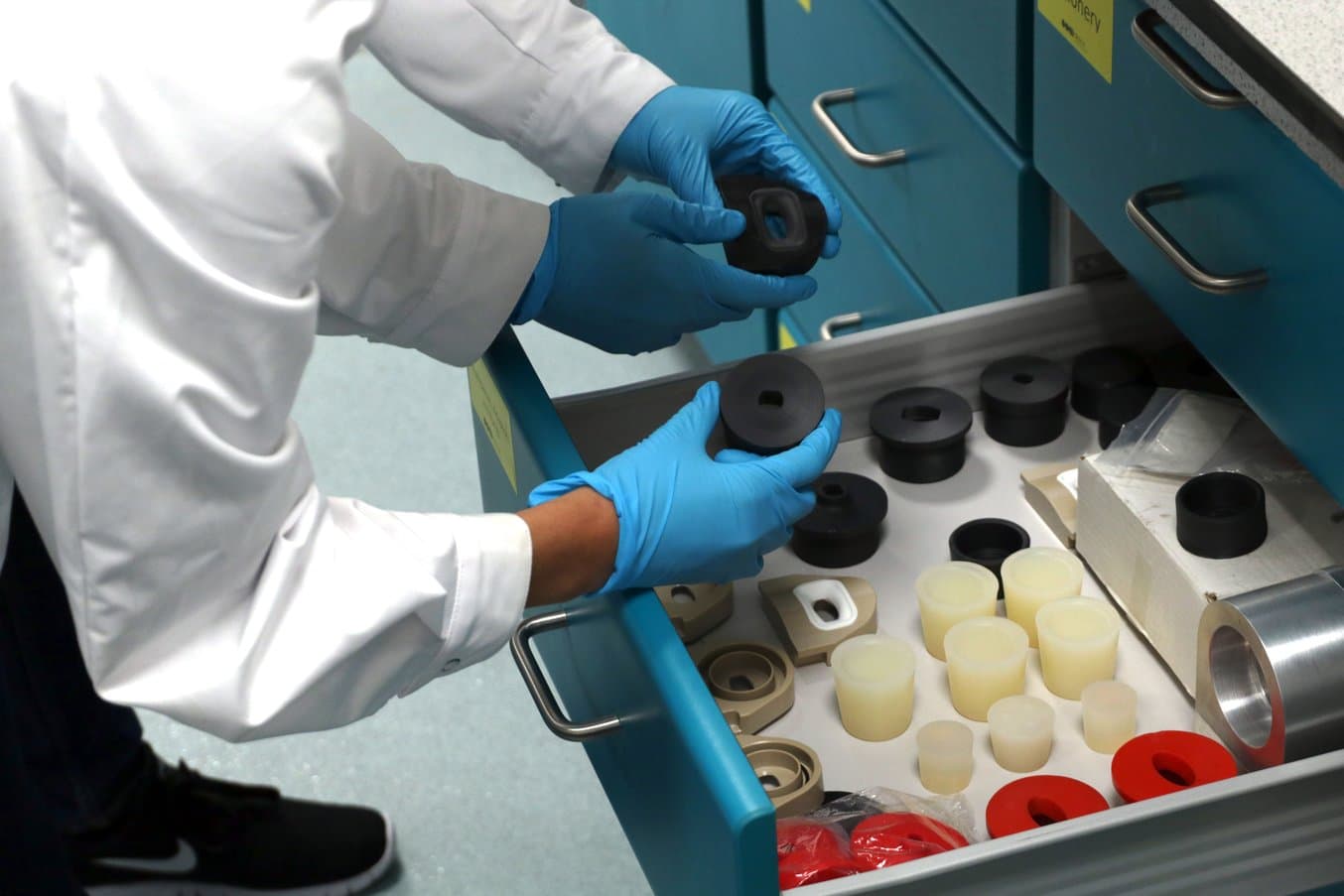
The team at Coalesce Product Development creates dozens of prototypes for each medical device.
According to the World Health Organization, an estimated 262 million people around the world suffer from asthma, leading to almost half a million deaths. For sufferers of breathing issues such as asthma or Chronic Obstructive Pulmonary Disease (COPD), an inhalation device is required to make breathing easier. Inhaled medication can control asthma symptoms and allow people with asthma to lead a normal, active life. Unfortunately, access and affordability are barriers for many, especially the uninsured and those in lower income countries. Studies published in the Annals of American Thoracic Society and the Journal of Allergy and Clinical Immunology estimated the annual costs per person for asthma treatment between $1,800 and $4,900 for inhalers and medications prescriptions alone, including direct costs (e.g. medicine and visits to the doctor) -- and indirect costs (e.g. time off from work). A 2005 Health Costs Survey found that 44% of all people with asthma stopped taking their medicine or visiting the doctor in an effort to save money.
UK-based medical device company Coalesce Product Development seeks to address some of these issues. The company develops novel, innovative drug delivery devices including inhalers and injectors, for use in generic inhalation products that offer significantly better value than brand name alternatives that can cost over $380 per month.
Inhalers need to be usable by a wide cross-section of society, from adolescents to elderly patients with comorbidities. Therefore, the precise size, shape, and user interface of each new inhaler needs to be very carefully designed and tested. In order to achieve this, the Coalesce team has turned to using in-house 3D printers to prototype, test, and create various devices in a multitude of different shapes and sizes. To test each product in development, the company also develops its own 3D printed test rigs, jigs, and fixtures.
In this post, we talked to Vinnay Chhabildas, Industrial Designer at Coalesce, to learn how the team has expanded its use of 3D printing over the past five years, why they continue to invest in Formlabs to develop their medical devices, and which resins they use for different applications.

Coalesce Product Development
Vinnay Chhabildas is using 3D printing to create ‘looks like’ prototypes, reduce outsourcing, and increase design iteration speed. The end result is innovative drug delivery devices.
Investing in Formlabs Ecosystem For Medical Device Manufacturing

Draft Resin is used to test fixtures before they are machined.

Coalesce first invested in the Form 2 stereolithography (SLA) 3D printer when it was released in 2015. At that time, the team was still heavily relying on outsourcing for prototypes, waiting days at a time for their parts to arrive in the mail. Before long, the team had multiple Form 2s and had significantly reduced their reliance on outsourcing.
Coalesce used the Form 2 to develop and prototype the crucial architecture for several inhaler and auto injector devices. For example, for caseworks of its electronic Breath Profiling Device (BPD), the designers chose White Resin for its smooth finish and mechanical properties, which allowed them to drill and add brass inserts.
Vinnay said at the time that “stereolithography offers a good balance of feature resolution, surface finish, durability, choice of materials, and dimensional accuracy. Because we develop devices with moving parts, we needed an effective way to prototype small mechanisms in-house. We kept an eye on advances in 3D printing technology over the past few years, and we liked the Formlabs approach. When the Form 2 was released, we ordered one immediately, followed by a second just a few weeks later.”
Initially, the 3D printed prototypes were used to develop the BPD’s exterior architecture. When the design was stable, the printed parts were painted and taken to the Drug Delivery to the Lungs (DDL) conference, an annual gathering of pulmonary and nasal drug delivery specialists. The BPD prototypes looked so realistic that they were often mistaken for a final, end-use product.
Eventually, the same device prototypes were used in a pulmonary function clinical study. The results showed how much variation can occur between different uninstructed inhalation profiles.
Using third party vendors would have cost approximately 20 times more than the raw cost of printing the parts in-house. The caseworks of the BPD cost £11 to produce on the Form 2 compared to around £250 when outsourced. According to Vinnay, however, the real benefit is the time saved: the parts took only eight hours to print and could be finished and painted within a few days. The same process would take an external contractor a week or two.
| Inhaler Casework | In-House 3D Printing | Outsourced 3D Printing |
|---|---|---|
| Cost | £11 | £250 |
| Lead Time | 1-2 Days | 1-2 Weeks |
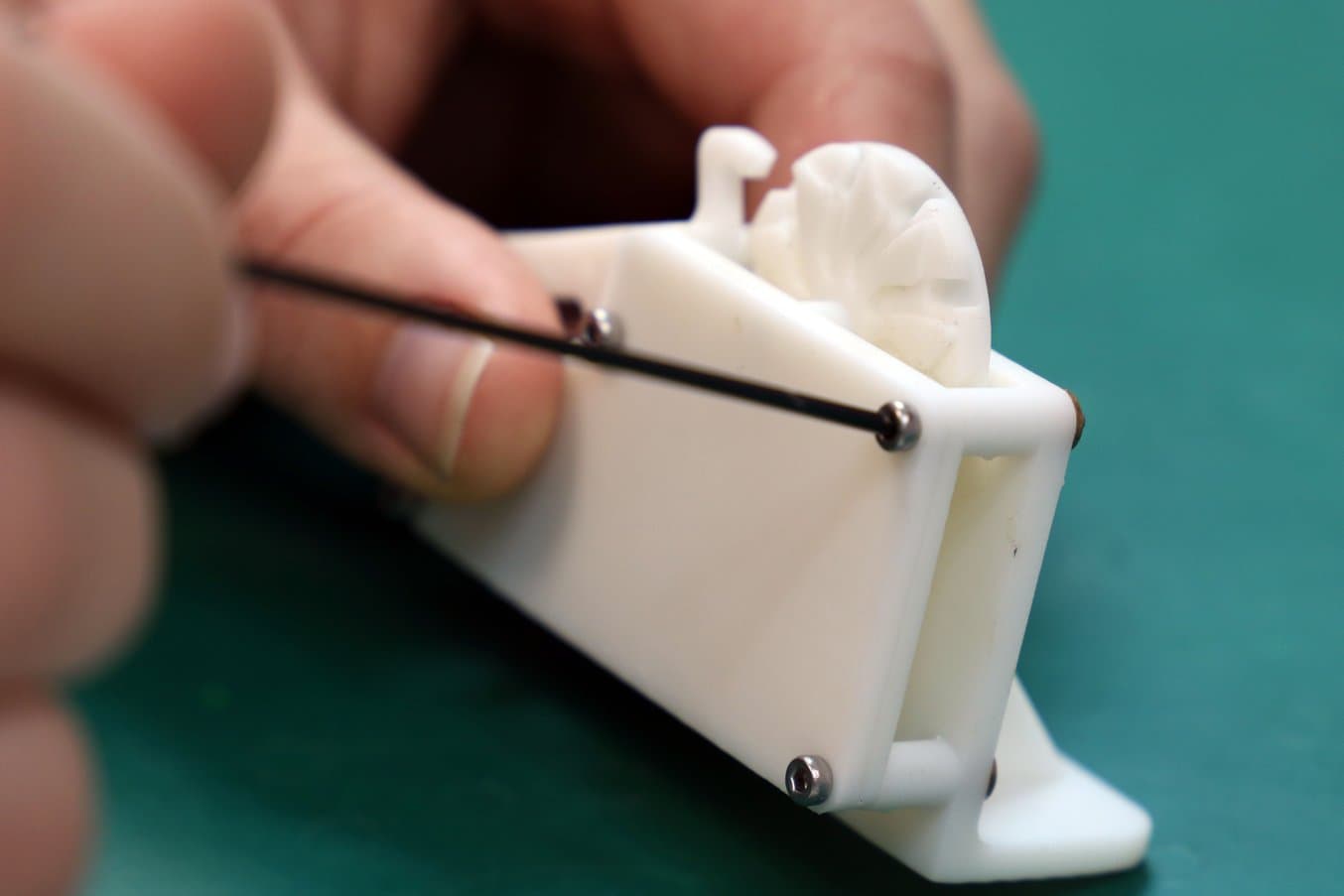
Screw threads can be easily inserted into 3D printed materials. Shown here, White Resin.
Expanding In-House 3D Printing With the Form 3
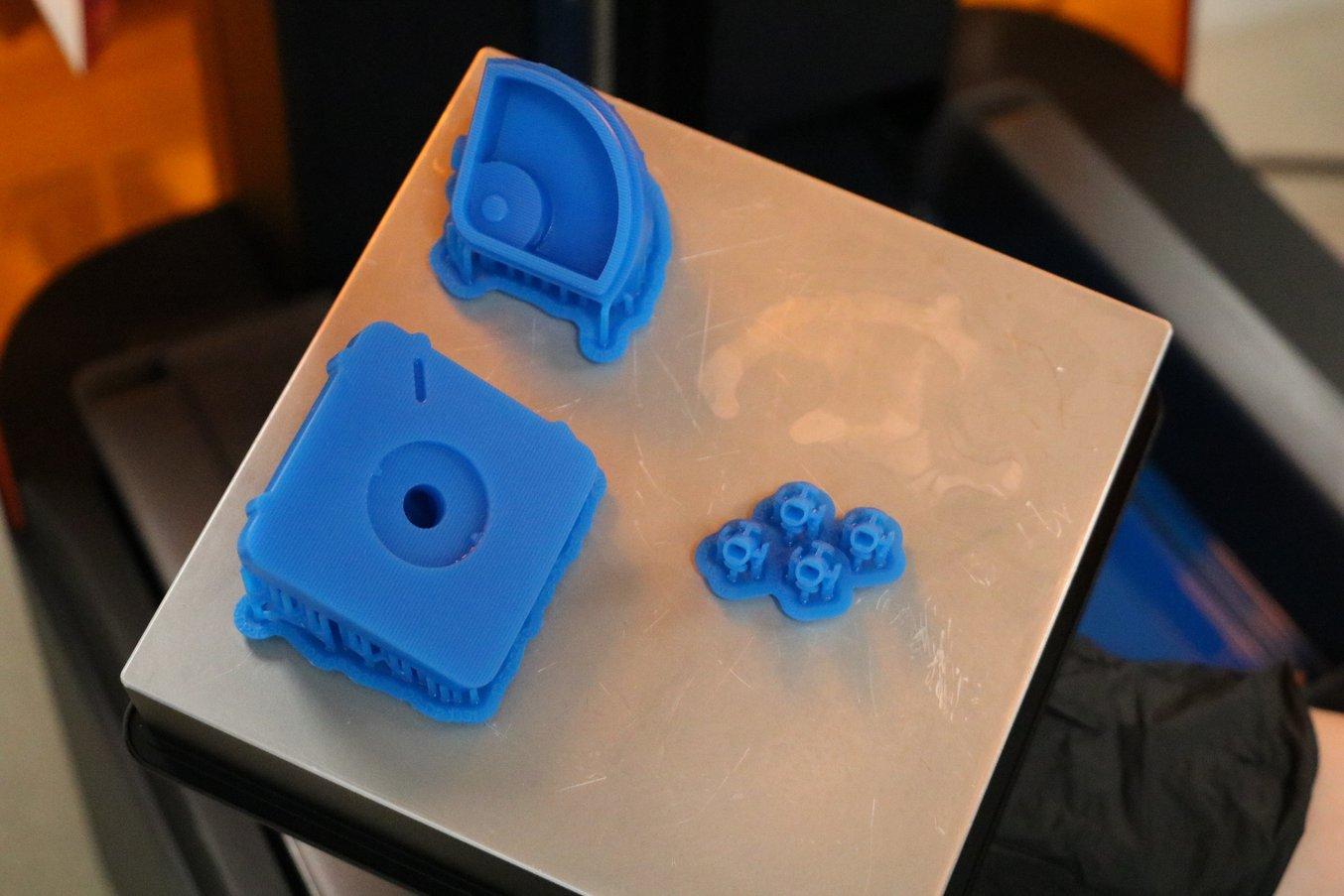
Rapid prototyping is vital to success at Coalesce.
After bringing most of their 3D printing in-house with the Form 2, and using the machines as workhorses for years, the team upgraded their fleet by purchasing three Form 3's.
With the Form 3, Coalesce was ready to move from purely aesthetics and looks-like prototypes, to actually integrating 3D printing into the development of their devices, including creating minute internal features. Due to the accuracy of Low-Force Stereolithography (LFS) 3D printing delivered by the Form 3, the team has been able to solve major problems facing inhaler development, notably analyzing fine particle distribution of the delivered dry powder formulations using analytical testing to ensure a smooth and accurate delivery of various drugs.
Vinnay put it succinctly, saying that “the Form 3 has allowed us to print fine features and delicate meshes, and to optimise the device during the design phase. We have the ability to model a part, change it on the fly, and have the physical part in a few hours. We can cut out the third-party supplier, and we get the parts more quickly. The Form 3s are absolutely essential to us. I can’t imagine not having them.”
Today, the team is running three Form 3s nonstop, five days per week, making them an inseparable part of the development process. Use cases for 3D printing have continued to expand, with the team now printing tooling to help test their devices. An example is printing different mouth pieces in BioMed Clear Resin, a biocompatible, skin safe material, for patient trials. 3D printing allows the team to skip the creation of expensive injection molded plastic parts.
Vinnay told us the reason his team has stuck with Formlabs is because “there is no better alternative to Formlabs printers on the market. There are other platforms but nothing that can provide the print quality, machine reliability and ease-of-use that we have become accustomed to with Formlabs. We are so well adapted to using PreForm and Dashboard, that anything else seems inferior. Since starting with the Form 2, and upgrading to the Form 3, we haven’t switched.”
A Vast Library of SLA 3D Printing Materials

Coalesce utilizes a wide range of Formlabs resins.
One of the major benefits of SLA 3D printing is the large materials library. This allows for a single Form 3 to play multiple roles inside an organization, printing different types of parts depending on the application.
In order to speed up the process of creating testing jigs, the team has turned to Draft Resin to create parts quicker. For example, fixtures print in approximately one hour with Draft Resin. The time saving is vital, with Vinnay telling us that “we also have a CNC machine in-house. Having the ability to test what the CNC part will look like in a 3D print, without having to set up the CNC machine, is invaluable.”
Coalesce utilizes the Formlabs material library in the following ways:
Draft Resin is used to test fixtures before they are machined. Due to the fast printing times, the team is able to quickly iterate between different fixture designs, changing fixture holes and screw mounts. By printing multiple fixtures per build platform, the team is able to accelerate the testing of their inhalers.
High Temp Resin is used to create tooling that works in conjunction with a heat-sealing tool. Since many of the inhaler parts must be heat-sealed together, having access to custom tooling to help control the process is vital. According to Vinnay, these parts are often not machinable, and 3D printing allows them to create the perfectly shaped tool.
Rigid Resin is also used for tooling. The team finds Rigid Resin is perfect for small, intricate features that need to maintain their dimensional accuracy, such as small mesh shapes that need to hold firm when subjected to airflow forces.
Grey Pro Resin is used in a similar manner to Rigid Resin, being used primarily for fixtures and tooling. The Coalesce team found that Grey Pro Resin held up better under wear and tear, such as gears meshing together or when there may be friction between parts.
Standard Resins (White Resin & Black Resin) are both used for aesthetic models. White Resin is great for exterior caseworks, as it is easy to paint. Fully painted and post-processed White Resin prints are used as presentation models before a device is greenlit for final production.
BioMed Clear Resin is used for printing mouthpieces that are sent off to be tested with real patients. Coalesce tests their devices through a Human Factors consultancy, and printing various mouthpieces in BioMed Clear Resin makes it more affordable to test a wide range of shapes with patients. Without BioMed Clear Resin, these plastic parts would have to be injected molded.
Elastic 50A Resin is mainly used in the chemical lab at Coalesce to create custom adapters which fit on a lung-simulation machine. The adapter has to seal reliably against the mouthpiece that is being tested, and so a silicone-like part is needed. Elastic 50A Resin can bend, stretch, compress, and hold up to repeated cycles without tearing, making it perfect for the adapters.
Clear Resin is used as a type of potting material due to its translucent appearance. The team will assemble versions of their devices in Clear Resin, polish the resin, and then observe how all of the various components come together inside the inhaler. This allows them to measure internal details that wouldn’t be visible when printing in Black Resin or Grey Pro Resin.
3D Printing for Medical Devices

The breath-actuated inhaler (BAI) builds on the features of the press-and-breathe device, but is easier to use; patients simply open-inhale-close.

The pre-metered dry powder inhaler (DPI) platform can accommodate multiple formulations, separately or in combination. It has an open-Inhale-close user-interface and a high-clarity dose-counter.
Deploying in-house 3D printing can drive immense value, and critical time advantages, for medical device development firms. Not only can it be used to create looks-like prototypes, it can also help to reduce outsourcing, increase design iteration speed, create vital tooling while eliminating the need for injection molding, and more.
Using Formlabs 3D printers, Coalesce Product Development shortened lead times for one medical device’s casework by 80–90 percent, and achieved 96 percent cost reduction. Over time they have continued to invest in and expand 3D printing applications, putting them on the leading edge of inhalation product development. The cost savings and speed of development have enabled the company to develop devices for its clients, and also to develop its own device technology for out-licensing to global pharmaceutical companies with relatively modest financial investments.
Ultimately, Vinnay summed up his experience using Formlabs printers in one sentence: “For Coalesce, the Formlabs 3D printers are vital.”
Contact Formlabs to learn how desktop SLA 3D printing can simplify prototyping and testing workflows and get a free sample for your specific application.


