BioMed Durable Resin
Medical
SLA
Biocompatibility
29.1 MPa
994 MPa
33 %
Select Printer Series and Resin Volume
| Bulk order quantity (L) | Discount % |
|---|---|
| 1 | 0% |
| 30 | 15% |
| 60 | 20% |
| 120 | 25% |
| 240 | 30% |
| 1,000 | 35% |
| 2,000 | 40% |
| 3,000 | 45% |
BioMed Durable Resin is manufactured in our FDA-registered, ISO 13485 certified facility and is also USP Class VI certified which makes it suitable for pharmaceutical and drug delivery applications.

BioMed Durable Resin has been added to your cart.
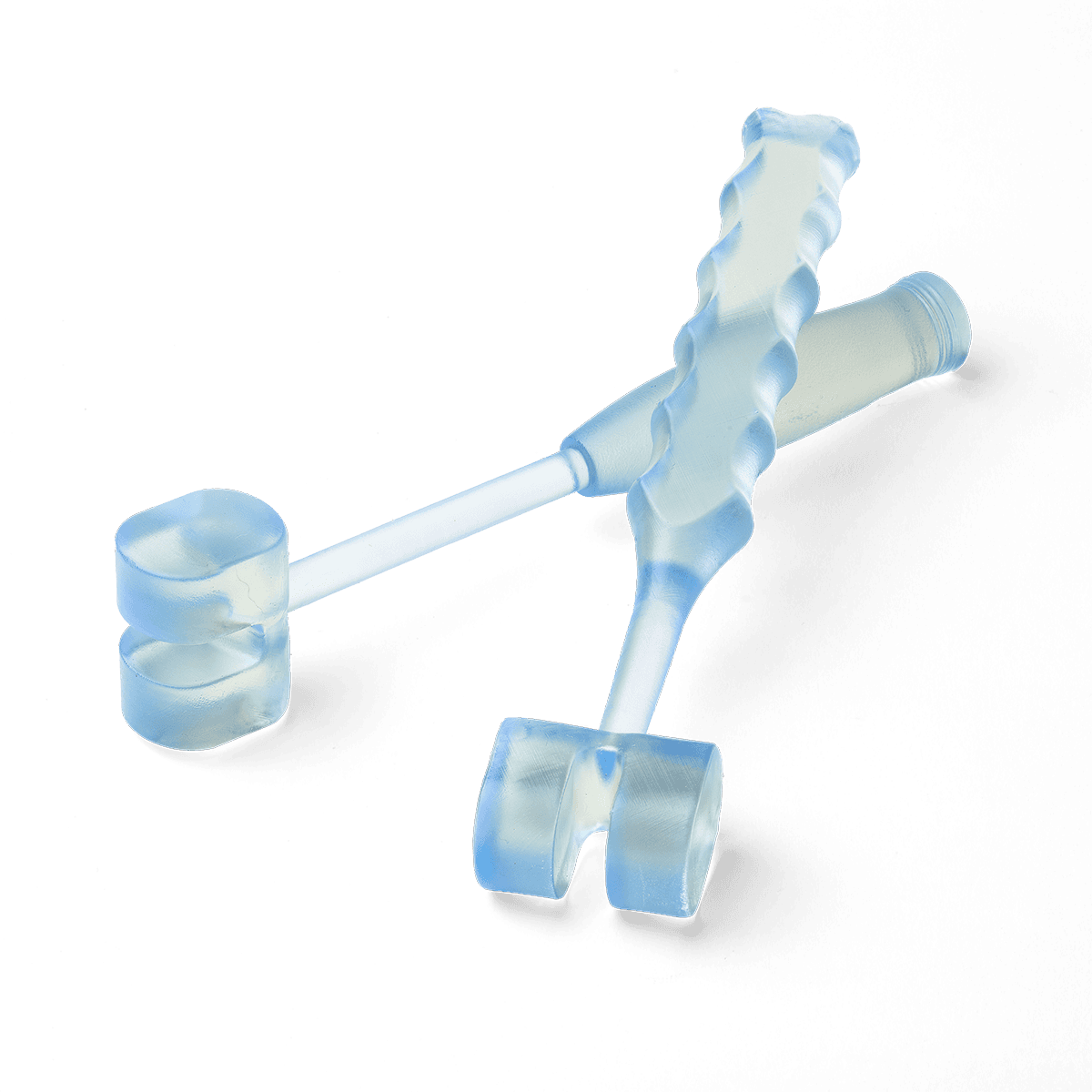
Why Choose BioMed Durable Resin?
Fuel medical innovation using 3D printing and BioMed Durable Resin for producing end-use devices where performance, impact resistance, and biocompatibility are critical.
Impact, Shatter, and Abrasion Resistant
Produce medical-grade parts that are impact resistant with a Notched Izod of 98 J/m.
Certified Medical-Grade Material
Leverage a material manufactured within our Quality Management Systems, with strict adherence to ISO 13485.
Biocompatible Parts
Common use case testing by Formlabs is intended to instill confidence that this material is suitable for biocompatible applications.
Excellent Clarity and Surface Finish
Parts produced using this material look and feel like end-use parts and have a very smooth surface finish.
Applications*
Introduce cutting-edge patient-specific instruments (PSI) and procedure-specific medical devices at the point of care. Improve patient outcomes, reduce complications, and reduce operating time with medical devices printed in BioMed Durable Resin.
BioMed Durable Resin is ideal for:
*Unlike Formlabs Dental Resins, no intended uses are provided for BioMed Resins. Material properties may vary based on part design, manufacturing practices, and other methods. It is the manufacturer’s responsibility and its respective customers and end-users to determine the biocompatibility and performance of all printed parts for their respective application and use. To instill confidence in potential users, Formlabs has tested biocompatibility for common use cases and manufactures BioMed Resins in an ISO 13485-certified facility. We can offer representative examples of what customers have done with our products and the supporting documentation we make freely available.

Formlabs manufactures our Biomed and Medical Device Resins at our FDA Registered facility in Ohio. These materials are designed and manufactured within our robust Quality Management System that is ISO 13485 and EU MDR certified. A dedicated team of operators and quality assurance professionals make the resins inside a certified ISO Class 8 clean room. All of our Medical Device Resins are appropriately registered with FDA and CE marked according to the EU MDR. View our ISO 13485 certification.
Material Properties*
BioMed Durable Resin
Ultimate Tensile Strength
Tensile Modulus
Elongation at Break
Flexural Strength
Flexural Modulus
Hardness Shore D
Notched Izod
Heat Deflection Temp @ 0.45 MPa
Post-Processing
Washing
BioMed Durable Resin may be washed using either a Formlabs-validated wash unit (1) or an ultrasonic wash unit (2).
The foundational step in any SLA post-processing workflow is to remove excess resin on the surface of the parts through an alcohol wash. Please refer to the material's manufacturing guide/IFU for washing instructions.
Curing
For biocompatible materials, post-curing is necessary to achieve the safety standards determined by regulatory agencies. Please follow the material’s manufacturing guide/IFU for proper post-processing.