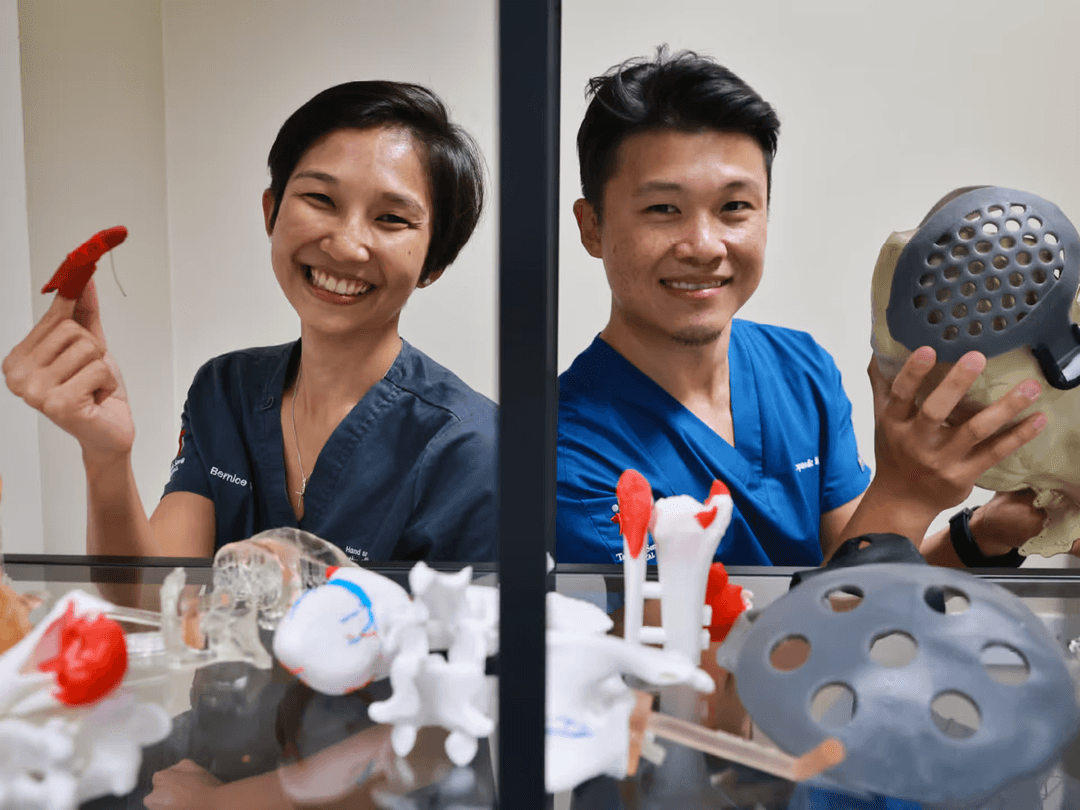
Formlabs Medical: Enabling Medical Device Firms and Health Systems to Advance the Standard of Care
The above model was 3D printed with Formlabs technology and used by a neurosurgeon to prepare for a complex aneurysm surgery.
3D printing is a vital tool in the future of precision medicine and medical device development. Advancements in 3D printing have made the technology more accessible and versatile, resulting in an increasing number of healthcare applications as demonstrated in hundreds of peer-reviewed publications and dozens of FDA-cleared devices. Founded in 2011 by three MIT researchers, when 3D printing was still inaccessible for most, Formlabs now has over 600 employees, a multi-billion dollar valuation, and a leading role in the 3D printing revolution in healthcare. While additive manufacturing technology is over thirty years old, Formlabs has quickly become the all-time best-selling professional 3D printing manufacturer, with over 75,000 printers shipped worldwide, including thousands in the medical community. From ventilation system components to prosthetics and surgical instruments, healthcare professionals and medical device engineers across the globe have relied on Formlabs Medical, its 3D printers and materials, and in-house QA/RA teams to quickly, safely, and affordably introduce new tools that improve the standard of care.
Formlabs helped pave the way for proven, accessible, and scalable 3D printing for healthcare professionals. In this post, we will cover the following:
- Why customers rely on Formlabs Medical
- Helping the healthcare industry print over 70 million parts
- The future of 3D printing in medicine
Why Customers Rely on Formlabs Medical
Formlabs was founded on the belief that anyone can make anything, an ethos we’ve embraced since day one. We design, develop, and manufacture printers, software, and materials and service a wide range of industries, equipped with the expertise to serve varying needs. Leveraging the expertise obtained while developing our initial machines and materials made for general engineering purposes, in 2016, we started investing heavily in dedicated solutions to serve the medical and dental community. Since that time, a countless number of medical devices have been developed with our machines, in addition to over a quarter million patients who have benefited from patient-specific, biocompatible surgical guides and appliances.
The Form 3B and Form 3BL are specifically designed for medical use: print patient-specific parts in a day at the point of care, or bring nimble, impactful R&D and low-volume commercial production in-house with advanced 3D printers optimized for biocompatible and engineering materials. We have made significant strides in developing biocompatible materials like BioMed Clear Resin and BioMed Amber Resin. Our technology has been validated in multiple FDA 510(k)s, ranging from surgical planning models and surgical guides to radiotherapy devices and sleep apnea devices. Our own products and manufacturing facilities also meet a wide range of international standards and hold several certifications relevant to their work.
A few Formlabs resources that our medical partners have come to rely on include:
- Medical Sales Teams and Medical Pro Service Plans in North America and Europe
- Biocompatible resins, including BioMed Clear (w/ USP Class VI, FDA MAF) and BioMed Amber, produced in our FDA-registered, ISO 13485 certified facility
- Expanded QA/RA team and Medical Sales Engineers to support you from your initial design and documentation to establishing a QMS and preparing regulatory filings. This team is complemented by a new Factory Solutions team to support operations and manufacturing at scale.
- Detailed sterilization compatibility and biocompatibility reports for six materials including Tough 1500 (SLA) and Nylon 12 (SLS).
To recap 2020 in particular, Formlabs Medical helped health systems and medical device companies make over 70 million COVID-19 NP swabs, received the first-ever FDA Emergency Use Authorization (EUA) issued to a 3D printing company, and launched a new line of biocompatible, sterilizable materials. Our own systems were challenged and we are proud of the resilience, agility, and expertise demonstrated by a countless number of employees. More details can be found in the following section.
Helping the Healthcare Industry Print Over 70 Million Parts
Medical Device Manufacturer Precision ADM uses over 100 printers to quickly produce 4 million swabs for Health Canada.
Formlabs has had a wide-reaching impact on the global medical community. Our 3D printers and materials have played an instrumental role in responding to the COVID-19 pandemic, helped with medical device manufacturing at scale, and assisted thousands of surgical operations, to name a few examples. Contact us for a list of the hundreds of peer-reviewed articles across a wide range of clinical and research applications that have leveraged Formlabs technology to date.
When the COVID-19 pandemic was declared a national emergency in the United States in March 2020, Formlabs didn’t hesitate to support clinical partners in testing and manufacturing solutions to address supply chain shortages. Our clinical partners produced personal protective equipment (PPE) components, test swabs, ventilator components, and more. We teamed up with academic medical centers (AMCs) across the United States, medical device firms in Singapore and Canada to address shortages across the globe. Precision ADM pivoted from 3D printing in metal to producing more than 100,000 swabs a week with their fleet of 100 Form 3Bs and has produced over 4 million swabs to date. The Formlabs Factory Solutions team—a team of multidisciplinary experts that support product ideation to print optimization and production implementation—supported Precision ADM as it scaled its printing capacity. Factory Solutions played an integral role from the start, working with Precision ADM to make their manufacturing process more efficient.
Beyond the pandemic, Formlabs has proven to be valuable and meet clinical accuracy requirements for a wide range of surgical operations. The workflow from CT/MRI to a printable model and deep dives into select applications can be found in our new Clinical Innovator video series. We are proud to have segmentation partners including GE Healthcare, Materialise Medical, and Axial3D to enable segmentation to be conducted at the point-of-care or outsourced, depending on the resources and preferences of the hospital.
From a regulatory perspective, thanks to an active, transparent, and collaborative stakeholder engagement process led by the Food and Drug Administration and Radiological Society of North America (RSNA) Special Interest Group (SIG) for 3D Printing, the regulatory environment has more clarity than ever before. The FDA published technical considerations for 3D printed medical devices in 2017 and released Current Practices and Regulations for patient-specific anatomic models. Anatomical models marketed for diagnostic use are considered medical devices and should use segmentation software with a 510(k) clearance. As of this writing, Formlabs has three printers validated by software vendors to produce such models. We continue to participate in the FDA stakeholder engagement process as it develops its Conceptual Framework for point-of-care medical device production.
Since patient-specific anatomical models and guides are not currently reimbursable at the national level, certain health systems have bundled them with other services or produced them at a direct loss outweighed by well-documented clinical gains and cost-avoidance implications. As a first step towards reimbursement, the American Medical Association (AMA) accepted Category III Current Procedural Terminology (CPT) codes for 3D anatomic modeling, which was led by the American College of Radiology (ACR). A joint RSNA-ACR 3D Printing Registry was also established to enable national data collection and analyses. As Formlabs sees benefit across our medical base and among RSNA SIG members, we’re proud to be an industry partner of the registry in the form of an unrestricted grant.
A few clinical uses of 3D printing can be found below:
- Preoperative planning for a complex sarcoma surgery.
- Creating and sizing custom-fit stent grafts for aortic aneurysms.
- Developing custom-knee braces and ankle foot orthoses.
Medical device firms and health systems that have publicly spoken about how Formlabs enables innovation from prototyping to production include:
- Restor3D, printing Single Use, Sterile Osteotomy Wedge Inserters for foot and ankle surgery.
- SlowWave, the first Oral Appliance Therapy (OAT) cleared by the FDA for snoring and mild to moderate obstructive sleep apnea.
- The US Department of Veterans Affairs (VA), printing an audiology stent to restore hearing to a veteran with a rare collapsed ear canal.
The Future of 3D Printing in Medicine
The future of medicine lies in customized, patient-specific care, more agile and responsive supply chains, and the ability to prototype and produce end-use parts with unprecedented speed. Formlabs is not only a leader in 3D printing, but also in 3D printing for the medical industry. With proven QA/RA and operations expertise, best-in-class hardware and materials, Formlabs is poised to help healthcare and medical professionals succeed from concept to commercialization or to the operating room.
To learn more about how we can help, please contact our dedicated medical 3D printing team. These team members have a track record in helping our medical customers succeed with 3D printing and would be proud to support your work.